FDA Approved Novel Drugs for 2020

FDA Approved Novel Drugs for 2020
FDA Approved Novel Drugs for 2020
What is Novel Drug?
A Novel Drug or a New Molecular Entity (NME) is an active, complex compound, which has not been previously approved by the FDA/EMA.
Every year, FDA’s Center for Drug Evaluation and Research (CDER) approves a wide range of new drugs and biological products.
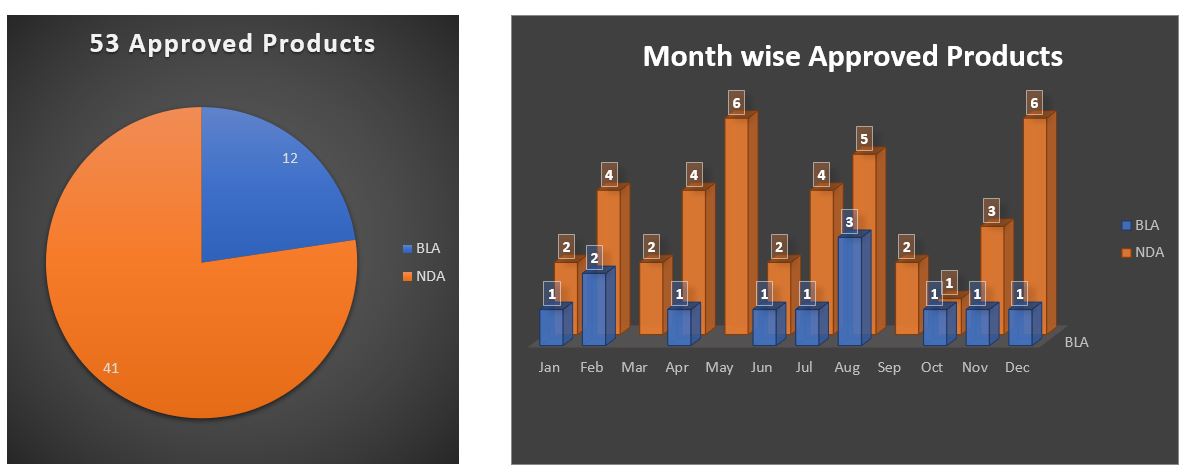
In 2020, FDA’s CDRER approved 53 new drugs, approved as new molecular entities (NMEs) for new drug applications (NDAs) or as new therapeutic biologics in connection with Biologics Licence Applications (BLAs).
New Drug Applications are 41
Biologics License Applications are 12
Some of these products are innovative new products that have never been used clinically.
Here is a list of new molecular entities and new therapeutic biological products approved by the CDRE in 2020.
The list does not contain vaccines, allergenic products, blood and blood products, plasma derivatives, cellular and gene therapy products, or other products approved in 2020 by the Center for Biologics Evaluation and Research.
FDA Approved Novel Drugs for 2020
| No. | Brand Name | Key Ingredient | Company Name | Approval | Approval Date | FDA-approved use |
| 1 | Ayvakit | avapritinib | Blueprint Medicines Corporation | NDA | 09 January 2020 | To treat adults with unresectable or metastatic gastrointestinal stromal tumor (GIST) |
| 2 | Tepezza | teprotumumab-trbw | Horizon Therapeutics Ireland DAC | BLA | 21 January 2020 | To treat Thyroid eye disease |
| 3 | Tazverik | tazemetostat | Epizyme | NDA | 23 January 2020 | To treat epithelioid sarcoma |
| 4 | Pizensy | lactitol | Braintree Laboratories, Inc | NDA | 12 February 2020 | To treat chronic idiopathic constipation (CIC) in adults |
| 5 | Nexletol | bempedoic acid | Esperion Therapeutics, Inc | NDA | 21 February 2020 | To treat adults with heterozygous familial hypercholesterolemia or established atherosclerotic cardiovascular disease who require additional lowering of LDL-C |
| 6 | Vyepti | eptinezumab-jjmr | Lundbeck Seattle BioPharmaceuticals, Inc. | BLA | 21 February 2020 | For the preventive treatment of migraine in adults |
| 7 | Barhemsys | amisulpride | Acacia Pharma Ltd | NDA | 26 February 2020 | To help prevent nausea and vomiting after surgery |
| 8 | Nurtec ODT | rimegepant | Biohaven Pharmaceuticals | NDA | 27 February 2020 | To treat migraine |
| 9 | Sarclisa | isatuximab | Sanofi Aventis US LLC. | BLA | 03 February 2020 | To treat multiple myloma |
| 10 | Isturisa | osilodrostat | Novartis Pharmaceuticals Corporation | NDA | 06 March 2020 | To treat adults with Cushing’s disease who either cannot undergo pituitary gland surgery or have undergone the surgery but still have the disease |
| 11 | Zeposia | ozanimod | Celgene Corporation | NDA | 25 March 2020 | To treat relapsing forms of multiple sclerosis |
| 12 | Koselugo | selumetinib | AstraZeneca | NDA | 10 April 2020 | To treat neurofibromatosis type 1, a genetic disorder of the nervous system causing tumors to grow on nerves |
| 13 | Tukysa | tucatinib | Seattle Genetics, Inc | NDA | 17 April 2020 | To treat advanced unresectable or metastatic HER2-positive breast cancer |
| 14 | Pemazyre | pemigatinib | Incyte Corporation | NDA | 17 April 2020 | To treat certain patients with cholangiocarcinoma, a rare form of cancer that forms in bile ducts |
| 15 | Trodelvy | sacituzumab govitecan-hziy | lmmunomedics, Inc | BLA | 22 April 2020 | To treat adult patients with metastatic triple-negative breast cancer who received at least two prior therapies for metastatic disease |
| 16 | Ongentys | opicapone | Neurocrine Biosciences, Inc | NDA | 24 April 2020 | To treat patients with Parkinson’s disease experiencing “off” episodes |
| 17 | Tabrecta | capmatinib | Novartis Pharmaceuticals Corporation | NDA | 06 May 2020 | To treat patients with non small cell lung cancer |
| 18 | Retevmo | selpercatinib | Loxo Oncology Inc., a wholly owned subsidiary of Eli Lilly and Company | NDA | 08 May 2020 | To treat lung and thyroid cancers |
| 19 | Qinlock | ripretinib | Deciphera Pharmaceuticals, LLC | NDA | 15 May 2020 | To treat advanced gastrointestinal-stromal tumors |
| 20 | Cerianna | fluoroestrdiol F18 | Zionexa-US Corporation | NDA | 20 May 2020 | Diagnostic imaging agent for certain patients with breast cancer |
| 21 | Artesunate | artesunate | Amivas, LLC | NDA | 26 May 2020 | To treat severe malaria |
| 22 | Tauvid | flortaucipir F18 | Avid Radiopharmaceuticals, Inc | NDA | 28 May 2020 | Diagnostic agent for patients with Alzheimer’s disease |
| 23 | Uplizna | inebilizumab-cdon | Viela Bio | BLA | 11 June 2020 | To treat neuromyelitis optica spectrum disorder |
| 24 | Zepzelca | lurbinectedin | Pharma Mar USA, Inc. | NDA | 15 June 2020 | To treat metastatic small cell lung cancer |
| 25 | Dojolvi | triheptanoin | Ultragenyx Pharmaceutical Inc. | NDA | 30 June 2020 | To treat molecularly long-chain fatty acid oxidation disorders |
| 26 | Byfavo | remimazolam | Cosmo Technologies, Ltd. | NDA | 02 July 2020 | For sedation |
| 27 | Rukobia | fostemsavir | ViiV Healthcare Company | NDA | 02 July 2020 | To treat HIV |
| 28 | Inqovi | decitabine and cedazuridine | Astex Pharmaceuticals, Inc. | NDA | 07 July 2020 | To treat adult patients with myelodysplastic syndromes |
| 29 | Xeglyze | abametapir | Dr. Reddy’s Laboratories | NDA | 24 July 2020 | To treat head lice |
| 30 | Monjuvi | tafasitamab-cxix | MorphoSys US Inc | BLA | 31 July 2020 | To treat relapsed or refractory diffuse large B-cell lymphoma |
| 31 | Blenrep | belantamab mafodotin-blmf | GlaxoSmithKline Intellectual Property Development Ltd. England | BLA | 05 August 2020 | To treat multiple myeloma |
| 32 | Lampit | nifurtimox | Bayer HealthCare Pharmaceuticals, Inc. | NDA | 06 August 2020 | To treat Chagas disease in certain pediatric patients younger than age 18 |
| 33 | Evrysdi | risdiplam | Genentech, Inc. | NDA | 07 August 2020 | To treat spinal muscular atrophy |
| 34 | Olinvyk | oliceridine | Trevena, Inc. | NDA | 07 August 2020 | To manage acute pain in certain adults |
| 35 | Viltepso | viltolarsen | Nippon Shinyaku Co., Ltd. | NDA | 12 August 2020 | To treat Duchenne muscular dystrophy |
| 36 | Enspryng | satralizumab-mwge | Genentech, Inc. | BLA | 14 August 2020 | To treat neuromyelitis optica spectrum disorder |
| 37 | Winlevi | clascoterone | Cassiopea SpA | NDA | 26 August 2020 | To treat acne |
| 38 | Sogroya | somapacitan-beco | Novo Nordisk Inc | BLA | 28 August 2020 | Growth hormone |
| 39 | Detectnet | copper Cu 64 dotatate injection | RadioMedix, Inc | NDA | 03 September 2020 | To help detect certain types of neuroendocrine tumors |
| 40 | Gavreto | pralsetinib | Blueprint Medicines Corporation | NDA | 04 September 2020 | To treat non-small lung cancer |
| 41 | Inmazeb | atoltivimab, maftivimab, and odesivimab-ebgn | Regeneron Pharmaceuticals, Inc | BLA | 14 October 2020 | To treat ebola virus |
| 42 | Veklury | remdesivir | Gilead Sciences, Inc. | NDA | 22 October 2020 | To treat COVID-19 |
| 43 | Zokinvy | lonafarnib | Eiger BioPharmaceuticals, Inc. | NDA | 20 November 2020 | To treat rare conditions related to premature aging |
| 44 | Oxlumo | lumasiran | Alnylam Pharmaceuticals, Inc | NDA | 23 November 2020 | To treat hyperoxaluria type 1 |
| 45 | Imcivree | setmelanotide | Rhythm Pharmaceuticals, Inc. | NDA | 25 November 2020 | To treat obesity and the control of hunger associated with pro-opiomelanocortin deficiency, a rare disorder that causes severe obesity that begins at an early age |
| 46 | Danyelza | naxitamab-gqgk | Y-MABS THERAPEUTICS INC | BLA | 25 November 2020 | To treat high-risk refractory or relapsed neuroblastoma |
| 47 | Gallium 68 PSMA-11 | Gallium 68 PSMA-11 | UNIV CA LOS ANGELES | NDA | 01 December 2020 | For detection and localization of prostate cancer |
| 48 | Orladeyo | berotralstat | BioCryst Pharmaceuticals, Inc. | NDA | 04 December 2020 | To treat patients with hereditary angioedema |
| 49 | Klisyri | tirbanibulin | Athenex, Inc | NDA | 14 December 2020 | To treat actinic Keratosis of the face or scalp |
| 50 | Margenza | margetuximab (anti-HER2 mAb) | MacroGenics, Inc | NDA | 16 December 2020 | To treat HER2+ breast cancer |
| 51 | Orgovyx | relugolix | Myovant Sciences GmbH | NDA | 18 December 2020 | To treat advanced prostate cancer |
| 52 | Ebanga | ansuvimab-zykl | Ridgeback Biotherapeutics, LP. | BLA | 21 December 2020 | To treat ebola |
| 53 | Gemtesa | viberon | Urovant Sciences | NDA | 23 December 2020 | To treat overactive bladder |
FDA Approved Novel Drugs for 2020: For more information: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2020