USPI label updates December 2020

USPI label updates December 2020
USPI label updates December 2020
The United States Prescription Information (USPI) is a key tool for sharing the benefits and risks of a prescription drug approved by the Food and Drug Administration (FDA).
In December 2020, approximately 91 Drug Safety-related Labeling Changes updated.
USPI label updates December 2020
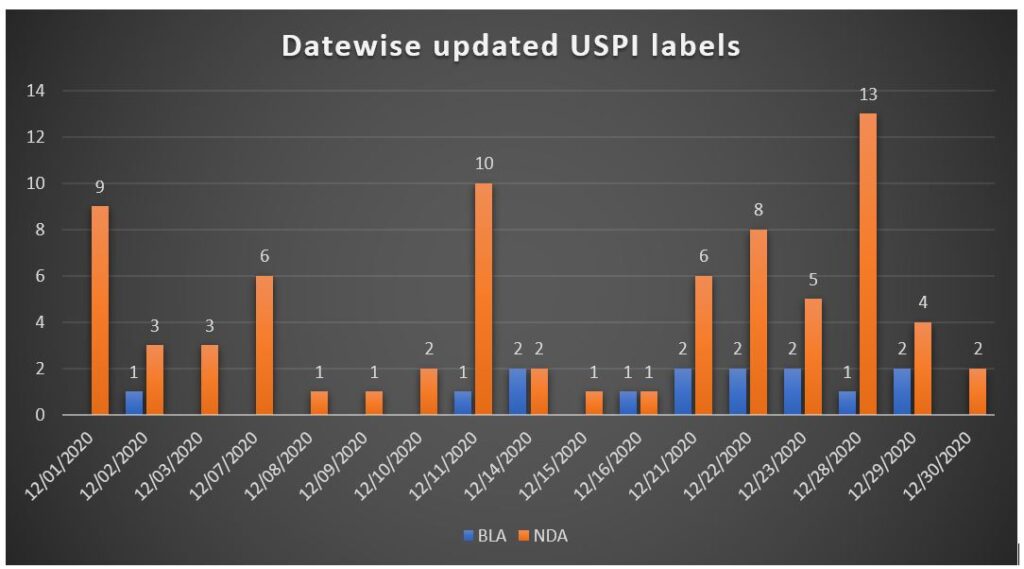
One or more subsequent sections have been updated in each product label update.
- Contraindications
- Warnings and Precautions
- Adverse Reactions
- Drug Interactions
- Boxed Warning
- Use in Special Populations
- Patient Counseling Information
- Patient Information
- Medication Guide
USPI label updates December 2020
| Drug Name | Active Ingredient | Company Name | Application Type | Database Updated |
| ZOVIRAX | ACYCLOVIR | Bausch Health Companies Inc | NDA | 12/28/2020 |
| ZILRETTA | TRIAMCINOLONE ACETONIDE | Flexion Therapeutics, Inc. | NDA | 12/07/2020 |
| ZEGERID | OMEPRAZOLE; SODIUM BICARBONATE | Salix Pharmaceuticals, Inc | NDA | 12/11/2020 |
| ZEGERID | OMEPRAZOLE; SODIUM BICARBONATE | Salix Pharmaceuticals, Inc | NDA | 12/11/2020 |
| ZAVESCA | MIGLUSTAT | Actelion Pharmaceuticals US, Inc | NDA | 12/11/2020 |
| ABILIFY MYCITE KIT | ARIPIPRAZOLE | Otsuka Pharmaceutical Co., Ltd | NDA | 12/30/2020 |
| ZANAFLEX | TIZANIDINE HYDROCHLORIDE | Covis Pharma | NDA | 12/23/2020 |
| YOSPRALA | ASPIRIN; OMEPRAZOLE | Genus Lifesciences Inc | NDA | 12/01/2020 |
| XPOVIO | SELINEXOR | Karyopharm Therapeutics Inc | NDA | 12/22/2020 |
| XOLAIR | OMALIZUMAB | Genentech, Inc/Novartis | BLA | 12/02/2020 |
| ACIPHEX SPRINKLE | RABEPRAZOLE SODIUM | Aytu Therapeutics, LLC | NDA | 12/01/2020 |
| Xeomin | incobotulinumtoxinA | Merz Pharmaceuticals | BLA | 12/22/2020 |
| XELPROS | LATANOPROST | Sun Pharmaceutical Industries, Inc | NDA | 12/28/2020 |
| VPRIV | VELAGLUCERASE ALFA | Shire Human Genetic Therapies, Inc | BLA | 12/11/2020 |
| VIZIMPRO | DACOMITINIB | Pfizer Labs | NDA | 12/29/2020 |
| VANTAS | HISTRELIN ACETATE | Endo Pharmaceuticals Inc | NDA | 12/23/2020 |
| TRUSOPT | DORZOLAMIDE HYDROCHLORIDE | Merck Sharp & Dohme Corp | NDA | 12/28/2020 |
| TESTIM | TESTOSTERONE | Endo Pharmaceuticals Inc | NDA | 12/02/2020 |
| TEFLARO | CEFTAROLINE FOSAMIL | Allergan USA, Inc. | NDA | 12/01/2020 |
| TECENTRIQ | ATEZOLIZUMAB | Genentech, Inc | BLA | 12/23/2020 |
| TASIGNA | NILOTINIB HYDROCHLORIDE MONOHYDRATE | Novartis Pharmaceuticals Corporation | NDA | 12/11/2020 |
| TALICIA | AMOXICILLIN; OMEPRAZOLE MAGNESIUM; RIFABUTIN | RedHill Biopharma Inc. | NDA | 12/01/2020 |
| TAGRISSO | OSIMERTINIB MESYLATE | AstraZeneca Pharmaceuticals LP | NDA | 12/22/2020 |
| SYNERA | LIDOCAINE; TETRACAINE | Galen US Inc | NDA | 12/21/2020 |
| SYMTUZA | COBICISTAT; DARUNAVIR ETHANOLATE; EMTRICITABINE; TENOFOVIR ALAFENAMIDE FUMARATE | Patheon Inc | NDA | 12/28/2020 |
| STELARA | USTEKINUMAB | Janssen Biotech, Inc | BLA | 12/14/2020 |
| STELARA | USTEKINUMAB | Janssen Biotech, Inc | BLA | 12/14/2020 |
| SAXENDA | LIRAGLUTIDE RECOMBINANT | Novo Nordisk | NDA | 12/10/2020 |
| RAPAFLO | SILODOSIN | Allergan USA, Inc. | NDA | 12/21/2020 |
| AMITIZA | LUBIPROSTONE | Sucampo Pharma Americas LLC | NDA | 12/09/2020 |
| AMMONUL | SODIUM BENZOATE; SODIUM PHENYLACETATE | f Bausch Health Companies Inc | NDA | 12/28/2020 |
| PROTONIX IV | PANTOPRAZOLE SODIUM | Wyeth Pharmaceuticals LLC | NDA | 12/01/2020 |
| ANTIZOL | FOMEPIZOLE | Orphan Medical | NDA | 12/28/2020 |
| PROHANCE MULTIPACK | GADOTERIDOL | BIPSO GmbH | NDA | 12/29/2020 |
| PROHANCE | GADOTERIDOL | BIPSO GmbH | NDA | 12/29/2020 |
| PREZISTA | DARUNAVIR ETHANOLATE | Janssen Pharmaceuticals | NDA | 12/28/2020 |
| PREZISTA | DARUNAVIR ETHANOLATE | Janssen Pharmaceuticals | NDA | 12/28/2020 |
| PREZCOBIX | COBICISTAT; DARUNAVIR ETHANOLATE | Janssen Ortho LLC | NDA | 12/28/2020 |
| ARCALYST | RILONACEPT | Regeneron Pharmaceuticals | BLA | 12/22/2020 |
| POMALYST | POMALIDOMIDE | Celgene Corporation | NDA | 12/07/2020 |
| PICATO | INGENOL MEBUTATE | LEO Laboratories Ltd | NDA | 12/21/2020 |
| PANTOPRAZOLE SODIUM | PANTOPRAZOLE SODIUM | Hikma Pharmaceuticals USA Inc. | NDA | 12/01/2020 |
| OMEPRAZOLE AND CLARITHROMYCIN AND AMOXICILLIN | AMOXICILLIN; CLARITHROMYCIN; OMEPRAZOLE | CUMBERLAND PHARMACEUTICALS INC | NDA | 12/01/2020 |
| OCREVUS | OCRELIZUMAB | Genentech, Inc | BLA | 12/16/2020 |
| NEXAVAR | SORAFENIB TOSYLATE | Bayer HealthCare Pharmaceuticals Inc. | NDA | 12/16/2020 |
| NEURONTIN | GABAPENTIN | Pfizer Inc | NDA | 12/22/2020 |
| NEURONTIN | GABAPENTIN | Pfizer Inc | NDA | 12/22/2020 |
| NEURONTIN | GABAPENTIN | Pfizer Inc | NDA | 12/22/2020 |
| MEPSEVII | VESTRONIDASE ALFA-VJBK | Ultragenyx Pharmaceutical Inc | BLA | 12/28/2020 |
| M.V.I. ADULT | ASCORBIC ACID; BIOTIN; CYANOCOBALAMIN; DEXPANTHENOL; ERGOCALCIFEROL; FOLIC ACID; NIACINAMIDE; PYRIDOXINE HYDROCHLORIDE; RIBOFLAVIN 5′-PHOSPHATE SODIUM; THIAMINE HYDROCHLORIDE; VITAMIN A; VITAMIN E; VITAMIN K | Hospira, Inc | NDA | 12/23/2020 |
| LYSTEDA | TRANEXAMIC ACID | Ferring Pharmaceuticals Inc | NDA | 12/11/2020 |
| LOTEMAX | LOTEPREDNOL ETABONATE | Bausch & Lomb Incorporated | NDA | 12/14/2020 |
| LOPID | GEMFIBROZIL | Pfizer Inc | NDA | 12/11/2020 |
| LIALDA | MESALAMINE | Takeda Pharmaceuticals | NDA | 12/21/2020 |
| LENVIMA | LENVATINIB MESYLATE | Eisai Inc | NDA | 12/22/2020 |
| KINERET | ANAKINRA | Swedish Orphan Biovitrum | BLA | 12/29/2020 |
| AUSTEDO | DEUTETRABENAZINE | Teva Pharmaceuticals | NDA | 12/03/2020 |
| AVALIDE | HYDROCHLOROTHIAZIDE; IRBESARTAN | sanofi-aventis U.S. LLC | NDA | 12/22/2020 |
| AVAPRO | IRBESARTAN | sanofi-aventis U.S. LLC | NDA | 12/22/2020 |
| KALBITOR | ECALLANTIDE | Dyax Corp | BLA | 12/29/2020 |
| JEVTANA KIT | CABAZITAXEL | sanofi-aventis U.S. LLC | NDA | 12/23/2020 |
| JANUVIA | SITAGLIPTIN PHOSPHATE | Merck Sharp & Dohme Corp. | NDA | 12/07/2020 |
| JANUMET XR | METFORMIN HYDROCHLORIDE; SITAGLIPTIN PHOSPHATE | Merck Sharp & Dohme Corp. | NDA | 12/08/2020 |
| JANUMET | METFORMIN HYDROCHLORIDE; SITAGLIPTIN PHOSPHATE | Merck Sharp & Dohme Corp. | NDA | 12/07/2020 |
| JALYN | DUTASTERIDE; TAMSULOSIN HYDROCHLORIDE | GlaxoSmithKline | NDA | 12/29/2020 |
| INVANZ | ERTAPENEM SODIUM | Merck Sharp & Dohme Corp. | NDA | 12/28/2020 |
| IMBRUVICA | IBRUTINIB | Pharmacyclics | NDA | 12/28/2020 |
| IMBRUVICA | IBRUTINIB | Pharmacyclics | NDA | 12/28/2020 |
| ICLUSIG | PONATINIB HYDROCHLORIDE | Takeda Pharmaceutical Co. Ltd | NDA | 12/23/2020 |
| HETLIOZ | TASIMELTEON | Vanda Pharmaceuticals Inc | NDA | 12/03/2020 |
| GENVOYA | COBICISTAT; ELVITEGRAVIR; EMTRICITABINE; TENOFOVIR ALAFENAMIDE FUMARATE | Gilead Sciences, Inc | NDA | 12/15/2020 |
| FINACEA | AZELAIC ACID | LEO Pharma Inc | NDA | 12/03/2020 |
| BENLYSTA | BELIMUMAB | GlaxoSmithKline | BLA | 12/21/2020 |
| BENLYSTA | BELIMUMAB | GlaxoSmithKline | BLA | 12/21/2020 |
| FINACEA | AZELAIC ACID | LEO Pharma Inc | NDA | 12/01/2020 |
| FERRLECIT | SODIUM FERRIC GLUCONATE COMPLEX | sanofi-aventis U.S. LLC | NDA | 12/07/2020 |
| ESOMEPRAZOLE STRONTIUM | ESOMEPRAZOLE STRONTIUM | Belcher Pharma Tech, LLC | NDA | 12/01/2020 |
| ERTACZO | SERTACONAZOLE NITRATE | Bausch Health US, LLC | NDA | 12/21/2020 |
| BICILLIN C-R | PENICILLIN G BENZATHINE; PENICILLIN G PROCAINE | Pfizer Inc | NDA | 12/11/2020 |
| BICILLIN C-R 900/300 | PENICILLIN G BENZATHINE; PENICILLIN G PROCAINE | Pfizer Inc | NDA | 12/11/2020 |
| BICILLIN L-A | PENICILLIN G BENZATHINE | Pfizer Inc | NDA | 12/11/2020 |
| EPIPEN | EPINEPHRINE | Mylan | NDA | 12/30/2020 |
| ENJUVIA | ESTROGENS, CONJUGATED SYNTHETIC B | Teva Pharmaceuticals | NDA | 12/02/2020 |
| ELEPSIA XR | LEVETIRACETAM | Sun Pharmaceutical Industries, Inc | NDA | 12/21/2020 |
| EFFIENT | PRASUGREL HYDROCHLORIDE | Daiichi Sankyo, Inc | NDA | 12/28/2020 |
| DEPO-SUBQ PROVERA 104 | MEDROXYPROGESTERONE ACETATE | Pfizer Inc | NDA | 12/14/2020 |
| DEPO-PROVERA | MEDROXYPROGESTERONE ACETATE | Pfizer Inc | NDA | 12/11/2020 |
| CENESTIN | ESTROGENS, CONJUGATED SYNTHETIC A | Teva Pharmaceuticals | NDA | 12/02/2020 |
| CEFUROXIME AND DEXTROSE IN DUPLEX CONTAINER | CEFUROXIME SODIUM | B. Braun Medical Inc | NDA | 12/10/2020 |
| CABLIVI | CAPLACIZUMAB-YHDP | Ablynx US | BLA | 12/23/2020 |
| BYDUREON BCISE | EXENATIDE | AstraZeneca | NDA | 12/07/2020 |
Here you can find November 2020 USPI label updates.