FDA Approved Drugs December 2020

FDA Approved Drugs December 2020
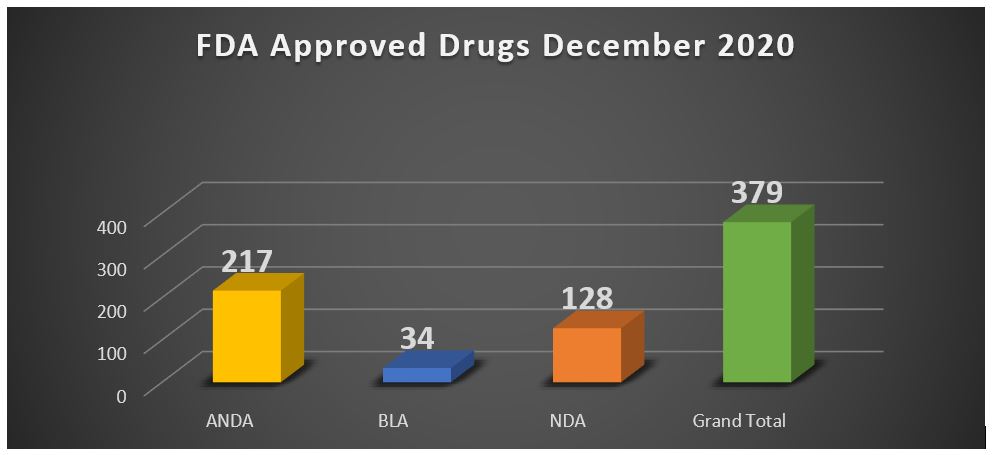
Here you can find FDA Approved Drugs in December 2020
This report includes NDAs, BLAs, ANDAs, approvals, and approved supplements to these requests, as well as interim ANDA/NDA approvals in 2020 December month.
This report excludes BLAs/NDAs and supplements to applications approved by CBER.
FDA Approved Drugs December 2020
| FDA Approval Date | Drug Name | Active Ingredients | Approval Type | Company | Submission Classification | Submission Status |
| 01 December 2020 | OMEPRAZOLE | OMEPRAZOLE | ANDA | LANNETT CO INC | Labeling | Approval |
| 01 December 2020 | ROPINIROLE HYDROCHLORIDE | ROPINIROLE HYDROCHLORIDE | ANDA | CELLTRION | Labeling | Approval |
| 01 December 2020 | LIDOCAINE | LIDOCAINE | ANDA | NOVEN PHARMS INC | Approval | |
| 01 December 2020 | DAUNORUBICIN HYDROCHLORIDE | DAUNORUBICIN HYDROCHLORIDE | ANDA | HISUN PHARM HANGZHOU | Manufacturing (CMC) | Approval |
| 01 December 2020 | DROXIDOPA | DROXIDOPA | ANDA | ALKEM LABS LTD | Tentative Approval | |
| 02 December 2020 | STAVUDINE | STAVUDINE | ANDA | MYLAN | Labeling | Approval |
| 02 December 2020 | STAVUDINE | STAVUDINE | ANDA | MYLAN | Labeling | Approval |
| 02 December 2020 | PREDNISONE INTENSOL | PREDNISONE | ANDA | HIKMA | Manufacturing (CMC) | Approval |
| 02 December 2020 | PENICILLAMINE | PENICILLAMINE | ANDA | GRANULES PHARMS | Approval | |
| 02 December 2020 | METOLAZONE | METOLAZONE | ANDA | ALEMBIC PHARMS LTD | Approval | |
| 02 December 2020 | VIGABATRIN | VIGABATRIN | ANDA | ALKEM LABS LTD | Approval | |
| 03 December 2020 | TACROLIMUS | TACROLIMUS | ANDA | MYLAN | Labeling | Approval |
| 03 December 2020 | TACROLIMUS | TACROLIMUS | ANDA | MYLAN | Labeling | Approval |
| 03 December 2020 | TACROLIMUS | TACROLIMUS | ANDA | MYLAN | Labeling | Approval |
| 03 December 2020 | TACROLIMUS | TACROLIMUS | ANDA | MYLAN | Labeling | Approval |
| 03 December 2020 | CELECOXIBA | CELECOXIB | ANDA | SCIEGEN PHARMS INC | Approval | |
| 03 December 2020 | KETOROLAC TROMETHAMINE | KETOROLAC TROMETHAMINE | ANDA | AUROBINDO PHARMA LTD | Approval | |
| 03 December 2020 | RITONAVIR | RITONAVIR | ANDA | HIKMA | Approval | |
| 03 December 2020 | MORPHINE SULFATE | MORPHINE SULFATE | ANDA | ALKEM LABS LTD | Approval | |
| 04 December 2020 | SUMATRIPTAN AND NAPROXEN SODIUM | NAPROXEN SODIUM; SUMATRIPTAN SUCCINATE | ANDA | SUN PHARM | Labeling | Approval |
| 04 December 2020 | CAPECITABINE | CAPECITABINE | ANDA | DR REDDYS LABS LTD | Approval | |
| 04 December 2020 | RIVASTIGMINE | RIVASTIGMINE | ANDA | ALVOGEN | Labeling | Approval |
| 04 December 2020 | CINACALCET HYDROCHLORIDE | CINACALCET HYDROCHLORIDE | ANDA | AUROBINDO PHARMA LTD | Labeling | Approval |
| 04 December 2020 | CINACALCET HYDROCHLORIDE | CINACALCET HYDROCHLORIDE | ANDA | AUROBINDO PHARMA LTD | Labeling | Approval |
| 04 December 2020 | CINACALCET HYDROCHLORIDE | CINACALCET HYDROCHLORIDE | ANDA | SUN PHARM | Labeling | Approval |
| 04 December 2020 | CINACALCET HYDROCHLORIDE | CINACALCET HYDROCHLORIDE | ANDA | SUN PHARM | Labeling | Approval |
| 04 December 2020 | CINACALCET HYDROCHLORIDE | CINACALCET HYDROCHLORIDE | ANDA | CIPLA | Labeling | Approval |
| 04 December 2020 | COLCHICINE | COLCHICINE | ANDA | DR REDDYS | Labeling | Approval |
| 04 December 2020 | CINACALCET HYDROCHLORIDE | CINACALCET HYDROCHLORIDE | ANDA | LUPIN LTD | Labeling | Approval |
| 04 December 2020 | CINACALCET HYDROCHLORIDE | CINACALCET HYDROCHLORIDE | ANDA | ALKEM LABS LTD | Labeling | Approval |
| 04 December 2020 | COLESEVELAM HYDROCHLORIDE | COLESEVELAM HYDROCHLORIDE | ANDA | INVENTIA | Approval | |
| 04 December 2020 | TETRABENAZINE | TETRABENAZINE | ANDA | AJANTA PHARMA LTD | Approval | |
| 04 December 2020 | DROXIDOPA | DROXIDOPA | ANDA | TASMAN PHARMA | Tentative Approval | |
| 07 December 2020 | FLUCONAZOLE IN SODIUM CHLORIDE 0.9% IN PLASTIC CONTAINER | FLUCONAZOLE | ANDA | WEST-WARD PHARMS INT | Labeling | Approval |
| 07 December 2020 | FLUCONAZOLE IN SODIUM CHLORIDE 0.9% IN PLASTIC CONTAINER | FLUCONAZOLE | ANDA | WEST-WARD PHARMS INT | Labeling | Approval |
| 07 December 2020 | ROPINIROLE HYDROCHLORIDE | ROPINIROLE HYDROCHLORIDE | ANDA | MYLAN | Labeling | Approval |
| 07 December 2020 | DEXMEDETOMIDINE HYDROCHLORIDE | DEXMEDETOMIDINE HYDROCHLORIDE | ANDA | AUROBINDO PHARMA LTD | Approval | |
| 07 December 2020 | DECITABINE | DECITABINE | ANDA | NIVAGEN PHARMS INC | Approval | |
| 08 December 2020 | ZAVESC | MIGLUSTAT | ANDA | ACTELION | Labeling | Approval |
| 08 December 2020 | FOSAPREPITANT DIMEGLUMINE | FOSAPREPITANT DIMEGLUMINE | ANDA | SANDOZ INC | Approval | |
| 08 December 2020 | ZOLEDRONIC | ZOLEDRONIC ACID | ANDA | GLAND PHARMA LTD | Labeling | Approval |
| 08 December 2020 | ZOLEDRONIC ACID | ZOLEDRONIC ACID | ANDA | GLAND PHARMA LTD | Labeling | Approval |
| 08 December 2020 | CYANOCOBALAMIN | CYANOCOBALAMIN | ANDA | AUROBINDO PHARMA LTD | Approval | |
| 08 December 2020 | DROXIDOPA | DROXIDOPA | ANDA | SUN PHARM INDS LTD | Tentative Approval | |
| 09 December 2020 | AMNESTEEM | ISOTRETINOIN | ANDA | MYLAN PHARMS INC | REMS | Approval |
| 09 December 2020 | CLARAVIS | ISOTRETINOIN | ANDA | TEVA PHARMS USA | REMS | Approval |
| 09 December 2020 | CLARAVIS | ISOTRETINOIN | ANDA | TEVA PHARMS USA | REMS | Approval |
| 09 December 2020 | MYORISAN | ISOTRETINOIN | ANDA | DOUGLAS PHARMS | REMS | Approval |
| 09 December 2020 | ZIDOVUDINE | ZIDOVUDINE | ANDA | AUROBINDO | Labeling | Approval |
| 09 December 2020 | OMEPRAZOLE | OMEPRAZOLE | ANDA | DR REDDYS LABS LTD | Labeling | Approval |
| 09 December 2020 | ZENATANE | ISOTRETINOIN | ANDA | DR REDDYS LABS LTD | REMS | Approval |
| 09 December 2020 | ISOTRETINOIN | ISOTRETINOIN | ANDA | AMNEAL PHARMS NY | REMS | Approval |
| 09 December 2020 | EVEROLIMUS | EVEROLIMUS | ANDA | PAR PHARM | Approval | |
| 09 December 2020 | FOSAPREPITANT DIMEGLUMINE | FOSAPREPITANT DIMEGLUMINE | ANDA | DR REDDYS | Approval | |
| 09 December 2020 | CLOBAZAM | CLOBAZAM | ANDA | CELLTRION | Approval | |
| 09 December 2020 | CELECOXIB | CELECOXIB | ANDA | ACIC PHARMS | Approval | |
| 09 December 2020 | MICAFUNGIN | MICAFUNGIN | ANDA | JIANGSU HANSOH PHARM | Tentative Approval | |
| 10 December 2020 | TRIAMTERENE AND HYDROCHLOROTHIAZIDE | HYDROCHLOROTHIAZIDE; TRIAMTERENE | ANDA | APOTEX INC | Labeling | Approval |
| 10 December 2020 | TRIAMTERENE AND HYDROCHLOROTHIAZIDE | HYDROCHLOROTHIAZIDE; TRIAMTERENE | ANDA | SANDOZ | Labeling | Approval |
| 10 December 2020 | TRIAMTERENE AND HYDROCHLOROTHIAZIDE | HYDROCHLOROTHIAZIDE; TRIAMTERENE | ANDA | SANDOZ | Labeling | Approval |
| 10 December 2020 | MOMETASONE FUROATE | MOMETASONE FUROATE | ANDA | ANDA REPOSITORY | Labeling | Approval |
| 10 December 2020 | IRBESARTAN | IRBESARTAN | ANDA | SANDOZ | Labeling | Approval |
| 10 December 2020 | MOMETASONE FUROATE | MOMETASONE FUROATE | ANDA | GLENMARK GENERICS | Labeling | Approval |
| 10 December 2020 | STIVARG | REGORAFENIB | ANDA | BAYER HLTHCARE | Manufacturing (CMC) | Approval |
| 10 December 2020 | TRANEXAMIC ACID | TRANEXAMIC ACID | ANDA | AUROBINDO PHARMA LTD | Labeling | Approval |
| 10 December 2020 | MECLIZINE HYDROCHLORIDE | MECLIZINE HYDROCHLORIDE | ANDA | INDICUS PHARMA | Labeling | Approval |
| 10 December 2020 | ASENAPINE MALEATE | ASENAPINE MALEATE | ANDA | BRECKENRIDGE | Approval | |
| 10 December 2020 | ASENAPINE MALEATE | ASENAPINE MALEATE | ANDA | BRECKENRIDGE | Tentative Approval | |
| 10 December 2020 | ASENAPINE MALEATE | ASENAPINE MALEATE | ANDA | ALEMBIC PHARMS LTD | Approval | |
| 10 December 2020 | ASENAPINE MALEATE | ASENAPINE MALEATE | ANDA | SIGMAPHARM LABS LLC | Approval | |
| 10 December 2020 | TRIAMTERENE AND HYDROCHLOROTHIAZIDE | HYDROCHLOROTHIAZIDE; TRIAMTERENE | ANDA | ZYDUS PHARMS | Labeling | Approval |
| 10 December 2020 | DOXYCYCLINE HYCLATE | DOXYCYCLINE HYCLATE | ANDA | NOSTRUM LABS INC | Approval | |
| 10 December 2020 | SODIUM NITROPRUSSIDE | SODIUM NITROPRUSSIDE | ANDA | AUROBINDO PHARMA LTD | Approval | |
| 10 December 2020 | TRANEXAMIC ACID | TRANEXAMIC ACID | ANDA | CAPLIN | Labeling | Approval |
| 10 December 2020 | OMEPRAZOLE | OMEPRAZOLE | ANDA | XIROMED | Approval | |
| 11 December 2020 | RISPERIDONE | RISPERIDONE | ANDA | ACTAVIS LABS FL INC | Labeling | Approval |
| 11 December 2020 | RISPERIDONE | RISPERIDONE | ANDA | ACTAVIS LABS FL INC | Labeling | Approval |
| 11 December 2020 | MESNA | MESNA | ANDA | SAGENT PHARMS INC | Labeling | Approval |
| 11 December 2020 | OMEPRAZOLE | OMEPRAZOLE | ANDA | BRECKENRIDGE | Labeling | Approval |
| 11 December 2020 | MEMANTINE HYDROCHLORIDE | MEMANTINE HYDROCHLORIDE | ANDA | POLYGEN PHARMS | Approval | |
| 11 December 2020 | GEMCITABINE HYDROCHLORIDE | GEMCITABINE HYDROCHLORIDE | ANDA | NANJING KING-FRIEND | Approval | |
| 11 December 2020 | MIDAZOLAM HYDROCHLORIDE | MIDAZOLAM HYDROCHLORIDE | ANDA | HIKMA | Approval | |
| 14 December 2020 | AZACITIDINE | AZACITIDINE | ANDA | ACCORD HLTHCARE | Labeling | Approval |
| 14 December 2020 | ABIRATERONE ACETATE | ABIRATERONE ACETATE | ANDA | MYLAN | Manufacturing (CMC) | Approval |
| 14 December 2020 | DARUNAVIR ETHANOLATE | DARUNAVIR ETHANOLATE | ANDA | MICRO LABS USA | Tentative Approval | |
| 14 December 2020 | LOPERAMIDE HYDROCHLORIDE AND SIMETHICONE | LOPERAMIDE HYDROCHLORIDE; SIMETHICONE | ANDA | AUROBINDO PHARMA LTD | Approval | |
| 14 December 2020 | AMINOCAPROIC ACID | AMINOCAPROIC ACID | ANDA | ANI PHARMS INC | Approval | |
| 14 December 2020 | CLOFARABINE | CLOFARABINE | ANDA | ACCORD HLTHCARE | Labeling | Approval |
| 15 December 2020 | SEVELAMER HYDROCHLORIDE | SEVELAMER HYDROCHLORIDE | ANDA | MYLAN | Approval | |
| 15 December 2020 | TERIFLUNOMIDE | TERIFLUNOMIDE | ANDA | MSN | Labeling | Approval |
| 15 December 2020 | TERIFLUNOMIDE | TERIFLUNOMIDE | ANDA | MSN | Labeling | Approval |
| 15 December 2020 | NICOTINE POLACRILEX | NICOTINE POLACRILEX | ANDA | DR REDDYS LABS SA | Labeling | Approval |
| 15 December 2020 | NICOTINE POLACRILEX | NICOTINE POLACRILEX | ANDA | DR REDDYS LABS SA | Labeling | Approval |
| 15 December 2020 | NICOTINE POLACRILEX | NICOTINE POLACRILEX | ANDA | DR REDDYS LABS SA | Labeling | Approval |
| 15 December 2020 | EPHEDRINE SULFATE | EPHEDRINE SULFATE | ANDA | HIKMA | Approval | |
| 16 December 2020 | SYNER | LIDOCAINE; TETRACAINE | ANDA | GALEN SPECIALTY | Labeling | Approval |
| 16 December 2020 | DULOXETINE HYDROCHLORIDE | DULOXETINE HYDROCHLORIDE | ANDA | AUROBINDO PHARMA LTD | Labeling | Approval |
| 16 December 2020 | DULOXETINE HYDROCHLORIDE | DULOXETINE HYDROCHLORIDE | ANDA | AUROBINDO PHARMA LTD | Labeling | Approval |
| 16 December 2020 | ACETYLCYSTEINE | ACETYLCYSTEINE | ANDA | AUROBINDO PHARMA LTD | Labeling | Approval |
| 16 December 2020 | METHYLPHENIDATE HYDROCHLORIDE | METHYLPHENIDATE HYDROCHLORIDE | ANDA | LANNETT CO INC | Approval | |
| 16 December 2020 | FLUDEOXYGLUCOSE F18 | FLUDEOXYGLUCOSE F-18 | ANDA | UNIV SOUTHERN CA | Approval | |
| 16 December 2020 | ETODOLAC | ETODOLAC | ANDA | BAYSHORE PHARMS LLC | Approval | |
| 16 December 2020 | EFINACONAZOLE | EFINACONAZOLE | ANDA | TEVA PHARMS USA | Approval | |
| 16 December 2020 | EFINACONAZOLE | EFINACONAZOLE | ANDA | PERRIGO PHARMA INTL | Approval | |
| 16 December 2020 | ICATIBANT ACETATE | ICATIBANT ACETATE | ANDA | NANG KUANG PHARM CO | Approval | |
| 16 December 2020 | EMPAGLIFLOZIN | EMPAGLIFLOZIN | ANDA | DR REDDYS LABS LTD | Tentative Approval | |
| 16 December 2020 | DOXYCYCLINE HYCLATE | DOXYCYCLINE HYCLATE | ANDA | EPIC PHARMA LLC | Approval | |
| 17 December 2020 | MEPERIDINE HYDROCHLORIDE | MEPERIDINE HYDROCHLORIDE | ANDA | EPIC PHARMA | Labeling | Approval |
| 17 December 2020 | MEPERIDINE HYDROCHLORIDE | MEPERIDINE HYDROCHLORIDE | ANDA | EPIC PHARMA | Labeling | Approval |
| 17 December 2020 | MEPERIDINE HYDROCHLORIDE | MEPERIDINE HYDROCHLORIDE | ANDA | EPIC PHARMA | Manufacturing (CMC) | Approval |
| 17 December 2020 | FLUVOXAMINE MALEATE | FLUVOXAMINE MALEATE | ANDA | APOTEX | Labeling | Approval |
| 17 December 2020 | FLUVOXAMINE MALEATE | FLUVOXAMINE MALEATE | ANDA | APOTEX | Labeling | Approval |
| 17 December 2020 | FOSPHENYTOIN SODIUM | FOSPHENYTOIN SODIUM | ANDA | DR REDDYS | Labeling | Approval |
| 17 December 2020 | FOSPHENYTOIN SODIUM | FOSPHENYTOIN SODIUM | ANDA | DR REDDYS | Labeling | Approval |
| 17 December 2020 | FOSPHENYTOIN SODIUM | FOSPHENYTOIN SODIUM | ANDA | DR REDDYS | Labeling | Approval |
| 17 December 2020 | ACETYLCYSTEINE | ACETYLCYSTEINE | ANDA | SAGENT PHARMS INC | Labeling | Approval |
| 17 December 2020 | EZETIMIBE AND SIMVASTATIN | EZETIMIBE; SIMVASTATIN | ANDA | MYLAN | Approval | |
| 17 December 2020 | COLESEVELAM HYDROCHLORIDE | COLESEVELAM HYDROCHLORIDE | ANDA | LUPIN LTD | Approval | |
| 17 December 2020 | FLUTICASONE PROPIONATE AND SALMETEROL XINAFOATE | FLUTICASONE PROPIONATE; SALMETEROL XINAFOATE | ANDA | HIKMA | Approval | |
| 17 December 2020 | OXYBUTYNIN CHLORIDE | OXYBUTYNIN CHLORIDE | ANDA | STRIDES PHARMA | Approval | |
| 17 December 2020 | DAPAGLIFLOZIN | DAPAGLIFLOZIN | ANDA | BIONPHARMA INC | Tentative Approval | |
| 18 December 2020 | CILOSTAZOL | CILOSTAZOL | ANDA | TEVA | Labeling | Approval |
| 18 December 2020 | MODAFINIL | MODAFINIL | ANDA | AUROBINDO PHARMA LTD | Labeling | Approval |
| 18 December 2020 | IMBRUVIC | IBRUTINIB | ANDA | PHARMACYCLICS INC | Efficacy | Approval |
| 18 December 2020 | OLOPATADINE HYDROCHLORIDE | OLOPATADINE HYDROCHLORIDE | ANDA | CIPLA | Labeling | Approval |
| 18 December 2020 | LENVIM | LENVATINIB MESYLATE | ANDA | EISAI INC | Labeling | Approval |
| 18 December 2020 | DABIGATRAN ETEXILATE MESYLATE | DABIGATRAN ETEXILATE MESYLATE | ANDA | GLENMARK PHARMS LTD | Tentative Approval | |
| 18 December 2020 | FINGOLIMOD HYDROCHLORIDE | FINGOLIMOD HYDROCHLORIDE | ANDA | APOTEX | Approval | |
| 18 December 2020 | FUROSEMIDE | FUROSEMIDE | ANDA | AREVA PHARMS | Approval | |
| 18 December 2020 | NALOXONE HYDROCHLORIDE | NALOXONE HYDROCHLORIDE | ANDA | PADDOCK LABS | Tentative Approval | |
| 18 December 2020 | MERZEE | ETHINYL ESTRADIOL; NORETHINDRONE ACETATE | ANDA | SLAYBACK PHARMA LLC | Approval | |
| 18 December 2020 | RUFINAMIDE | RUFINAMIDE | ANDA | LUPIN LTD | Approval | |
| 18 December 2020 | HYDRALAZINE HYDROCHLORIDE | HYDRALAZINE HYDROCHLORIDE | ANDA | HIKMA | Approval | |
| 18 December 2020 | ETOMIDATE | ETOMIDATE | ANDA | CAPLIN | Approval | |
| 21 December 2020 | TRILYTE | POLYETHYLENE GLYCOL 3350; POTASSIUM CHLORIDE; SODIUM BICARBONATE; SODIUM CHLORIDE | ANDA | MYLAN | Labeling | Approval |
| 21 December 2020 | GRANISETRON HYDROCHLORIDE | GRANISETRON HYDROCHLORIDE | ANDA | BAXTER HLTHCARE CORP | Labeling | Approval |
| 21 December 2020 | PEG-3350, POTASSIUM CHLORIDE, SODIUM BICARBONATE, SODIUM CHLORIDE | POLYETHYLENE GLYCOL 3350; POTASSIUM CHLORIDE; SODIUM BICARBONATE; SODIUM CHLORIDE | ANDA | NOVEL LABS INC | Labeling | Approval |
| 21 December 2020 | BUPROPION HYDROCHLORIDE | BUPROPION HYDROCHLORIDE | ANDA | MYLAN | Labeling | Approval |
| 21 December 2020 | BUPROPION HYDROCHLORIDE | BUPROPION HYDROCHLORIDE | ANDA | MYLAN | Labeling | Approval |
| 21 December 2020 | BUPROPION HYDROCHLORIDE | BUPROPION HYDROCHLORIDE | ANDA | MYLAN | Labeling | Approval |
| 21 December 2020 | ZOLEDRONIC ACID | ZOLEDRONIC ACID | ANDA | SAGENT PHARMS INC | Labeling | Approval |
| 21 December 2020 | ZOLEDRONIC ACID | ZOLEDRONIC ACID | ANDA | SAGENT PHARMS INC | Labeling | Approval |
| 21 December 2020 | ZOLEDRONIC ACID | ZOLEDRONIC ACID | ANDA | SAGENT PHARMS INC | Labeling | Approval |
| 21 December 2020 | ZOLEDRONIC ACID | ZOLEDRONIC ACID | ANDA | SAGENT PHARMS INC | Labeling | Approval |
| 21 December 2020 | BUPROPION HYDROCHLORIDE | BUPROPION HYDROCHLORIDE | ANDA | YICHANG HUMANWELL | Labeling | Approval |
| 21 December 2020 | SUCCINYLCHOLINE CHLORIDE | SUCCINYLCHOLINE CHLORIDE | ANDA | DEVA HOLDING AS | Approval | |
| 22 December 2020 | AMPICILLIN TRIHYDRATE | AMPICILLIN/AMPICILLIN TRIHYDRATE | ANDA | SANDOZ | Labeling | Approval |
| 22 December 2020 | IFOSFAMIDE | IFOSFAMIDE | ANDA | TEVA PHARMS USA | Labeling | Approval |
| 22 December 2020 | PRAMIPEXOLE DIHYDROCHLORIDE | PRAMIPEXOLE DIHYDROCHLORIDE | ANDA | ZYDUS PHARMS USA INC | Labeling | Approval |
| 22 December 2020 | PRAMIPEXOLE DIHYDROCHLORIDE | PRAMIPEXOLE DIHYDROCHLORIDE | ANDA | SANDOZ INC | Labeling | Approval |
| 22 December 2020 | PRAMIPEXOLE DIHYDROCHLORIDE | PRAMIPEXOLE DIHYDROCHLORIDE | ANDA | SANDOZ INC | Labeling | Approval |
| 22 December 2020 | PRAMIPEXOLE DIHYDROCHLORIDE | PRAMIPEXOLE DIHYDROCHLORIDE | ANDA | SANDOZ INC | Labeling | Approval |
| 22 December 2020 | ABACAVIR SULFATE, LAMIVUDINE AND ZIDOVUDINE | ABACAVIR SULFATE; LAMIVUDINE; ZIDOVUDINE | ANDA | LUPIN LTD | Labeling | Approval |
| 22 December 2020 | ABACAVIR SULFATE, LAMIVUDINE AND ZIDOVUDINE | ABACAVIR SULFATE; LAMIVUDINE; ZIDOVUDINE | ANDA | LUPIN LTD | Labeling | Approval |
| 22 December 2020 | ABACAVIR SULFATE, LAMIVUDINE AND ZIDOVUDINE | ABACAVIR SULFATE; LAMIVUDINE; ZIDOVUDINE | ANDA | LUPIN LTD | Labeling | Approval |
| 22 December 2020 | ABACAVIR SULFATE, LAMIVUDINE AND ZIDOVUDINE | ABACAVIR SULFATE; LAMIVUDINE; ZIDOVUDINE | ANDA | LUPIN LTD | Labeling | Approval |
| 22 December 2020 | AMBRISENTAN | AMBRISENTAN | ANDA | WATSON LABS INC | REMS | Approval |
| 22 December 2020 | AMBRISENTAN | AMBRISENTAN | ANDA | SIGMAPHARM LABS LLC | REMS | Approval |
| 22 December 2020 | AMBRISENTAN | AMBRISENTAN | ANDA | MYLAN | REMS | Approval |
| 22 December 2020 | AMBRISENTAN | AMBRISENTAN | ANDA | PAR PHARM INC | REMS | Approval |
| 22 December 2020 | AMBRISENTAN | AMBRISENTAN | ANDA | ZYDUS PHARMS | REMS | Approval |
| 22 December 2020 | AMBRISENTAN | AMBRISENTAN | ANDA | CIPLA | REMS | Approval |
| 22 December 2020 | AMBRISENTAN | AMBRISENTAN | ANDA | SUN PHARM | REMS | Approval |
| 22 December 2020 | EFINACONAZOLE | EFINACONAZOLE | ANDA | LUPIN LTD | Tentative Approval | |
| 22 December 2020 | BIVALIRUDIN | BIVALIRUDIN | ANDA | HAINAN POLY PHARMACEUTICAL CO LTD | Tentative Approval | |
| 23 December 2020 | FLUOXETINE HYDROCHLORIDE | FLUOXETINE HYDROCHLORIDE | ANDA | TEVA PHARMS USA | Labeling | Approval |
| 23 December 2020 | FLUOXETINE HYDROCHLORIDE | FLUOXETINE HYDROCHLORIDE | ANDA | TEVA PHARMS USA | Labeling | Approval |
| 23 December 2020 | FLUOXETINE HYDROCHLORIDE | FLUOXETINE HYDROCHLORIDE | ANDA | TEVA PHARMS USA | Labeling | Approval |
| 23 December 2020 | VINORELBINE TARTRATE | VINORELBINE TARTRATE | ANDA | TEVA PHARMS USA | Labeling | Approval |
| 23 December 2020 | FOMEPIZOLE | FOMEPIZOLE | ANDA | AM REGENT | Labeling | Approval |
| 23 December 2020 | FOMEPIZOLE | FOMEPIZOLE | ANDA | NAVINTA LLC | Labeling | Approval |
| 23 December 2020 | FOMEPIZOLE | FOMEPIZOLE | ANDA | MYLAN INSTITUTIONAL | Labeling | Approval |
| 23 December 2020 | FOSPHENYTOIN SODIUM | FOSPHENYTOIN SODIUM | ANDA | MYLAN LABS LTD | Labeling | Approval |
| 23 December 2020 | FENTANYL CITRATE | FENTANYL CITRATE | ANDA | SPECGX LLC | REMS | Approval |
| 23 December 2020 | FENTANYL CITRATE | FENTANYL CITRATE | ANDA | WATSON LABS | REMS | Approval |
| 23 December 2020 | BUPROPION HYDROCHLORIDE | BUPROPION HYDROCHLORIDE | ANDA | ACTAVIS LABS FL INC | Labeling | Approval |
| 23 December 2020 | BUPROPION HYDROCHLORIDE | BUPROPION HYDROCHLORIDE | ANDA | ACTAVIS LABS FL INC | Labeling | Approval |
| 23 December 2020 | BUPROPION HYDROCHLORIDE | BUPROPION HYDROCHLORIDE | ANDA | ACTAVIS LABS FL INC | Labeling | Approval |
| 23 December 2020 | APREPITANT | APREPITANT | ANDA | SANDOZ | Labeling | Approval |
| 23 December 2020 | APREPITANT | APREPITANT | ANDA | SANDOZ | Labeling | Approval |
| 23 December 2020 | ACYCLOVIR | ACYCLOVIR | ANDA | CADILA PHARMS LTD | Labeling | Approval |
| 23 December 2020 | TACROLIMUS | TACROLIMUS | ANDA | BELCHER | Labeling | Approval |
| 23 December 2020 | FLUOXETINE HYDROCHLORIDE | FLUOXETINE HYDROCHLORIDE | ANDA | CADILA PHARMS LTD | Labeling | Approval |
| 23 December 2020 | FENTANYL CITRATE | FENTANYL CITRATE | ANDA | ACTAVIS LABS FL INC | REMS | Approval |
| 23 December 2020 | FENOFIBRIC ACID | CHOLINE FENOFIBRATE | ANDA | YICHANG HUMANWELL | Approval | |
| 28 December 2020 | IFOSFAMIDE | IFOSFAMIDE | ANDA | WEST-WARD PHARMS INT | Labeling | Approval |
| 28 December 2020 | SEVELAMER CARBONATE | SEVELAMER CARBONATE | ANDA | INVAGEN PHARMS | Labeling | Approval |
| 28 December 2020 | MILNACIPRAN HYDROCHLORIDE | MILNACIPRAN HYDROCHLORIDE | ANDA | MYLAN PHARMS INC | Tentative Approval | |
| 28 December 2020 | NEBIVOLOL HYDROCHLORIDE | NEBIVOLOL HYDROCHLORIDE | ANDA | MICRO LABS LTD | Tentative Approval | |
| 28 December 2020 | AMLODIPINE AND OLMESARTAN MEDOXOMIL | AMLODIPINE BESYLATE; OLMESARTAN MEDOXOMIL | ANDA | AUROBINDO PHARMA LTD | Labeling | Approval |
| 28 December 2020 | AMLODIPINE AND OLMESARTAN MEDOXOMIL | AMLODIPINE BESYLATE; OLMESARTAN MEDOXOMIL | ANDA | ALEMBIC PHARMS LTD | Labeling | Approval |
| 28 December 2020 | AMLODIPINE AND OLMESARTAN MEDOXOMIL | AMLODIPINE BESYLATE; OLMESARTAN MEDOXOMIL | ANDA | MICRO LABS | Labeling | Approval |
| 28 December 2020 | GLUCAGON | GLUCAGON | ANDA | AMPHASTAR PHARMS INC | Approval | |
| 28 December 2020 | LURASIDONE HYDROCHLORIDE | LURASIDONE HYDROCHLORIDE | ANDA | PIRAMAL HLTHCARE UK | Approval | |
| 28 December 2020 | ENALAPRIL MALEATE | ENALAPRIL MALEATE | ANDA | BIONPHARMA INC | Tentative Approval | |
| 29 December 2020 | BENZPHETAMINE HYDROCHLORIDE | BENZPHETAMINE HYDROCHLORIDE | ANDA | IMPAX LABS | Labeling | Approval |
| 29 December 2020 | TADALAFIL | TADALAFIL | ANDA | GLENMARK PHARMS LTD | Approval | |
| 29 December 2020 | NEBIVOLOL | NEBIVOLOL | ANDA | AUROBINDO PHARMA LTD | Tentative Approval | |
| 29 December 2020 | APREMILAST | APREMILAST | ANDA | GLENMARK PHARMS LTD | Tentative Approval | |
| 29 December 2020 | EMPAGLIFLOZIN | EMPAGLIFLOZIN | ANDA | AIZANT | Tentative Approval | |
| 29 December 2020 | GABAPENTIN ENCARBIL | GABAPENTIN ENCARBIL | ANDA | GLENMARK PHARMS LTD | Tentative Approval | |
| 29 December 2020 | RIFAXIMIN | RIFAXIMIN | ANDA | SANDOZ INC | Tentative Approval | |
| 29 December 2020 | POTASSIUM CHLORIDE | POTASSIUM CHLORIDE | ANDA | ASCENT PHARMS INC | Approval | |
| 29 December 2020 | HEPARIN SODIUM | HEPARIN SODIUM | ANDA | BE PHARMS | Approval | |
| 29 December 2020 | HEPARIN SODIUM | HEPARIN SODIUM | ANDA | BE PHARMS | Approval | |
| 30 December 2020 | DULOXETINE HYDROCHLORIDE | DULOXETINE HYDROCHLORIDE | ANDA | HETERO LABS LTD III | Labeling | Approval |
| 30 December 2020 | DULOXETINE HYDROCHLORIDE | DULOXETINE HYDROCHLORIDE | ANDA | HETERO LABS LTD III | Manufacturing (CMC) | Approval |
| 30 December 2020 | DULOXETINE HYDROCHLORIDE | DULOXETINE HYDROCHLORIDE | ANDA | HETERO LABS LTD III | Labeling | Approval |
| 30 December 2020 | LEVOFLOXACIN IN DEXTROSE 5% IN PLASTIC CONTAINER | LEVOFLOXACIN | ANDA | GLAND PHARMA LTD | Approval | |
| 30 December 2020 | FINGOLIMOD HYDROCHLORIDE | FINGOLIMOD HYDROCHLORIDE | ANDA | ALKEM LABS LTD | Approval | |
| 31 December 2020 | PIMTREA | DESOGESTREL; ETHINYL ESTRADIOL | ANDA | NOVAST LABS | Labeling | Approval |
| 31 December 2020 | XULANE | ETHINYL ESTRADIOL; NORELGESTROMIN | ANDA | MYLAN TECHNOLOGIES | Labeling | Approval |
| 31 December 2020 | BUPROPION HYDROCHLORIDE | BUPROPION HYDROCHLORIDE | ANDA | SUN PHARM | Approval | |
| 31 December 2020 | OLANZAPINE | OLANZAPINE | ANDA | PHARMTAK INC | Approval | |
| 31 December 2020 | OLANZAPINE | OLANZAPINE | ANDA | PHARMTAK INC | Approval | |
| 31 December 2020 | CLINDAMYCIN PHOSPHATE | CLINDAMYCIN PHOSPHATE | ANDA | GAGE DEVELOPMENT | Approval | |
| 31 December 2020 | PIRFENIDONE | PIRFENIDONE | ANDA | SANDOZ INC | Tentative Approval |
Do you want to know FDA Approved Novel Drugs for 2020. Click here