FDA Approved Drugs January 2021

FDA Approved Drugs January 2021
Here you can find FDA Approved Drugs in January 2021
This report includes NDAs, BLAs, ANDAs, approvals, and approved supplements to these requests, as well as interim ANDA/NDA approvals in January month 2021.
This report excludes BLAs/NDAs and supplements to applications approved by CBER.
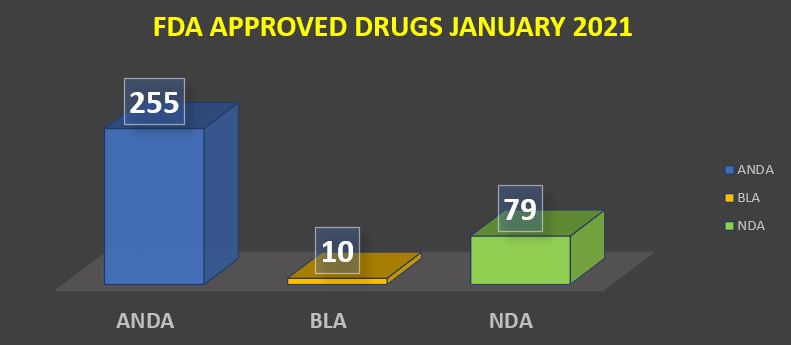
FDA Approved Drugs January 2021
| Drug Name | Approval Type | Active Ingredients | Company | Submission Classification | Submission Status | Approval Date |
| BUPROPION HYDROCHLORIDE | ANDA | BUPROPION HYDROCHLORIDE | IMPAX LABS | Labeling | Approval | 01/04/2021 |
| BUPROPION HYDROCHLORIDE | ANDA | BUPROPION HYDROCHLORIDE | IMPAX LABS | Labeling | Approval | 01/04/2021 |
| BUPROPION HYDROCHLORIDE | ANDA | BUPROPION HYDROCHLORIDE | IMPAX LABS | Labeling | Approval | 01/04/2021 |
| BUPROPION HYDROCHLORIDE | ANDA | BUPROPION HYDROCHLORIDE | IMPAX LABS | Labeling | Approval | 01/04/2021 |
| BUPROPION HYDROCHLORIDE | ANDA | BUPROPION HYDROCHLORIDE | IMPAX LABS | Labeling | Approval | 01/04/2021 |
| BUPROPION HYDROCHLORIDE | ANDA | BUPROPION HYDROCHLORIDE | WATSON LABS INC | Labeling | Approval | 01/04/2021 |
| BUPROPION HYDROCHLORIDE | ANDA | BUPROPION HYDROCHLORIDE | WATSON LABS INC | Labeling | Approval | 01/04/2021 |
| MOMETASONE FUROATE | ANDA | MOMETASONE FUROATE | COSETTE | Labeling | Approval | 01/04/2021 |
| TEMOZOLOMIDE | ANDA | TEMOZOLOMIDE | ANI PHARMS INC | Labeling | Approval | 01/04/2021 |
| TEMOZOLOMIDE | ANDA | TEMOZOLOMIDE | LANNETT CO INC | Labeling | Approval | 01/04/2021 |
| TETRABENAZINE | ANDA | TETRABENAZINE | HETERO LABS LTD V | Labeling | Approval | 01/04/2021 |
| TETRABENAZINE | ANDA | TETRABENAZINE | SUN PHARM | Labeling | Approval | 01/04/2021 |
| BUPROPION HYDROCHLORIDE | ANDA | BUPROPION HYDROCHLORIDE | INVAGEN PHARMS | Labeling | Approval | 01/04/2021 |
| BUPROPION HYDROCHLORIDE | ANDA | BUPROPION HYDROCHLORIDE | INVAGEN PHARMS | Labeling | Approval | 01/04/2021 |
| BUPROPION HYDROCHLORIDE | ANDA | BUPROPION HYDROCHLORIDE | INVAGEN PHARMS | Labeling | Approval | 01/04/2021 |
| AMLODIPINE AND OLMESARTAN MEDOXOMIL | ANDA | AMLODIPINE BESYLATE; OLMESARTAN MEDOXOMIL | MACLEODS PHARMS LTD | Labeling | Approval | 01/04/2021 |
| SEVELAMER CARBONATE | ANDA | SEVELAMER CARBONATE | AMNEAL PHARMS CO | Labeling | Approval | 01/04/2021 |
| AMLODIPINE AND OLMESARTAN MEDOXOMIL | ANDA | AMLODIPINE BESYLATE; OLMESARTAN MEDOXOMIL | GLENMARK PHARMS LTD | Labeling | Approval | 01/04/2021 |
| SOLIFENACIN SUCCINATE | ANDA | SOLIFENACIN SUCCINATE | AMNEAL PHARMS CO | Labeling | Approval | 01/04/2021 |
| CYANOCOBALAMIN | ANDA | CYANOCOBALAMIN | SANDOZ INC | Approval | 01/04/2021 | |
| SEVELAMER CARBONATE | ANDA | SEVELAMER CARBONATE | ANXIN | Labeling | Approval | 01/04/2021 |
| BENZPHETAMINE HYDROCHLORIDE | ANDA | BENZPHETAMINE HYDROCHLORIDE | SPECGX LLC | Labeling | Approval | 01/05/2021 |
| BENZPHETAMINE HYDROCHLORIDE | ANDA | BENZPHETAMINE HYDROCHLORIDE | KVK TECH | Labeling | Approval | 01/05/2021 |
| NEUPOGEN | BLA | FILGRASTIM | AMGEN | Approval | 01/05/2021 | |
| NEULASTA | BLA | PEGFILGRASTIM | AMGEN | Approval | 01/05/2021 | |
| LIDOCAINE | ANDA | LIDOCAINE | DR REDDYS | Approval | 01/05/2021 | |
| SULFAMETHOXAZOLE AND TRIMETHOPRIM | ANDA | SULFAMETHOXAZOLE; TRIMETHOPRIM | LUPIN LTD | Approval | 01/05/2021 | |
| SITAGLIPTIN PHOSPHATE | ANDA | SITAGLIPTIN PHOSPHATE | AJANTA PHARMA LTD | Tentative Approval | 01/05/2021 | |
| EXPAREL | NDA | BUPIVACAINE | PACIRA PHARMS INC | Labeling | Approval | 01/06/2021 |
| OLANZAPINE AND FLUOXETINE HYDROCHLORIDE | ANDA | FLUOXETINE HYDROCHLORIDE; OLANZAPINE | SANDOZ | Labeling | Approval | 01/06/2021 |
| OLANZAPINE AND FLUOXETINE HYDROCHLORIDE | ANDA | FLUOXETINE HYDROCHLORIDE; OLANZAPINE | SANDOZ | Labeling | Approval | 01/06/2021 |
| OLANZAPINE AND FLUOXETINE HYDROCHLORIDE | ANDA | FLUOXETINE HYDROCHLORIDE; OLANZAPINE | SANDOZ | Labeling | Approval | 01/06/2021 |
| OLANZAPINE AND FLUOXETINE HYDROCHLORIDE | ANDA | FLUOXETINE HYDROCHLORIDE; OLANZAPINE | SANDOZ | Labeling | Approval | 01/06/2021 |
| BENZPHETAMINE HYDROCHLORIDE | ANDA | BENZPHETAMINE HYDROCHLORIDE | EPIC PHARMA LLC | Labeling | Approval | 01/06/2021 |
| BENZPHETAMINE HYDROCHLORIDE | ANDA | BENZPHETAMINE HYDROCHLORIDE | ANDA REPOSITORY | Labeling | Approval | 01/06/2021 |
| OMEGA-3-ACID ETHYL ESTERS | ANDA | OMEGA-3-ACID ETHYL ESTERS | APOTEX | Labeling | Approval | 01/06/2021 |
| OMEGA-3-ACID ETHYL ESTERS | ANDA | OMEGA-3-ACID ETHYL ESTERS | TEVA PHARMS USA | Labeling | Approval | 01/06/2021 |
| BENZPHETAMINE HYDROCHLORIDE | ANDA | BENZPHETAMINE HYDROCHLORIDE | EMCURE PHARMS LTD | Labeling | Approval | 01/06/2021 |
| SOLIFENACIN SUCCINATE | ANDA | SOLIFENACIN SUCCINATE | WATSON LABS INC | Labeling | Approval | 01/06/2021 |
| OMEGA-3-ACID ETHYL ESTERS | ANDA | OMEGA-3-ACID ETHYL ESTERS | AMNEAL PHARMS | Labeling | Approval | 01/06/2021 |
| FOSAPREPITANT DIMEGLUMINE | ANDA | FOSAPREPITANT DIMEGLUMINE | FRESENIUS KABI USA | Labeling | Approval | 01/06/2021 |
| FOSAPREPITANT DIMEGLUMINE | ANDA | FOSAPREPITANT DIMEGLUMINE | FRESENIUS KABI USA | Labeling | Approval | 01/06/2021 |
| OMEGA-3-ACID ETHYL ESTERS | ANDA | OMEGA-3-ACID ETHYL ESTERS | BIONPHARMA INC | Labeling | Approval | 01/06/2021 |
| OMEGA-3-ACID ETHYL ESTERS | ANDA | OMEGA-3-ACID ETHYL ESTERS | ASCENT PHARMS INC | Labeling | Approval | 01/06/2021 |
| TETRABENAZINE | ANDA | TETRABENAZINE | MYLAN | Labeling | Approval | 01/06/2021 |
| QVAR REDIHALER | NDA | BECLOMETHASONE DIPROPIONATE | NORTON WATERFORD | Labeling | Approval | 01/06/2021 |
| ALOGLIPTIN | ANDA | ALOGLIPTIN | SUNSHINE LAKE | Tentative Approval | 01/06/2021 | |
| OMEGA-3-ACID ETHYL ESTERS | ANDA | OMEGA-3-ACID ETHYL ESTERS | PURACAP PHARM LLC | Labeling | Approval | 01/06/2021 |
| OMEGA-3-ACID ETHYL ESTERS | ANDA | OMEGA-3-ACID ETHYL ESTERS | ZYDUS | Labeling | Approval | 01/06/2021 |
| FOSAPREPITANT DIMEGLUMINE | ANDA | FOSAPREPITANT DIMEGLUMINE | LUPIN LTD | Labeling | Approval | 01/06/2021 |
| IVERMECTIN | ANDA | IVERMECTIN | TARO | Labeling | Approval | 01/06/2021 |
| OMEGA-3-ACID ETHYL ESTERS | ANDA | OMEGA-3-ACID ETHYL ESTERS | SOFGEN PHARMS | Labeling | Approval | 01/06/2021 |
| FOSAPREPITANT DIMEGLUMINE | ANDA | FOSAPREPITANT DIMEGLUMINE | SUNGEN PHARMA | Labeling | Approval | 01/06/2021 |
| OMEGA-3-ACID ETHYL ESTERS | ANDA | OMEGA-3-ACID ETHYL ESTERS | GLW | Labeling | Approval | 01/06/2021 |
| LEVOTHYROXINE SODIUM | ANDA | LEVOTHYROXINE SODIUM | TEVA PHARMS USA INC | Approval | 01/06/2021 | |
| EMPAGLIFLOZIN;METFORMIN HYDROCHLORIDE | ANDA | EMPAGLIFLOZIN;METFORMIN HYDROCHLORIDE | LUPIN LTD | Tentative Approval | 01/06/2021 | |
| RISPERDAL CONSTA | NDA | RISPERIDONE | JANSSEN PHARMS | Labeling | Approval | 01/07/2021 |
| CADUET | NDA | AMLODIPINE BESYLATE; ATORVASTATIN CALCIUM | PHARMACIA | Labeling | Approval | 01/07/2021 |
| CHILDREN’S CLARITIN | NDA | LORATADINE | BAYER HEALTHCARE LLC | Labeling | Approval | 01/07/2021 |
| CLARITIN | NDA | LORATADINE | BAYER HEALTHCARE LLC | Labeling | Approval | 01/07/2021 |
| AZATHIOPRINE SODIUM | ANDA | AZATHIOPRINE SODIUM | WEST-WARD PHARMS INT | Labeling | Approval | 01/07/2021 |
| BROMOCRIPTINE MESYLATE | ANDA | BROMOCRIPTINE MESYLATE | SANDOZ INC | Labeling | Approval | 01/07/2021 |
| MIRTAZAPINE | ANDA | MIRTAZAPINE | APOTEX INC | Labeling | Approval | 01/07/2021 |
| PHENYLEPHRINE HYDROCHLORIDE | ANDA | PHENYLEPHRINE HYDROCHLORIDE | AUROBINDO PHARMA LTD | Approval | 01/07/2021 | |
| TADALAFIL | ANDA | TADALAFIL | SUNSHINE | Labeling | Approval | 01/07/2021 |
| NUBEQA | NDA | DAROLUTAMIDE | BAYER HEALTHCARE | Efficacy | Approval | 01/07/2021 |
| PENTAMIDINE ISETHIONATE | ANDA | PENTAMIDINE ISETHIONATE | EMCURE PHARMS LTD | Approval | 01/07/2021 | |
| FLUPHENAZINE HYDROCHLORIDE | ANDA | FLUPHENAZINE HYDROCHLORIDE | CEROVENE INC | Approval | 01/07/2021 | |
| LORATADINE | ANDA | LORATADINE | UNIQUE PHARM | Approval | 01/07/2021 | |
| BIVALIRUDIN IN 0.9% SODIUM CHLORIDE | NDA | BIVALIRUDIN | BAXTER HLTHCARE CORP | Labeling | Approval | 01/08/2021 |
| PEMETREXED | NDA | PEMETREXED | HOSPIRA INC | Type 3 – New Dosage Form | Tentative Approval | 01/08/2021 |
| CLARITIN HIVES RELIEF REDITAB | NDA | LORATADINE | BAYER HEALTHCARE LLC | Labeling | Approval | 01/11/2021 |
| CLARITIN REDITABS | NDA | LORATADINE | BAYER HEALTHCARE LLC | Labeling | Approval | 01/11/2021 |
| BUPROPION HYDROCHLORIDE | ANDA | BUPROPION HYDROCHLORIDE | SANDOZ | Labeling | Approval | 01/11/2021 |
| BUPROPION HYDROCHLORIDE | ANDA | BUPROPION HYDROCHLORIDE | SANDOZ | Labeling | Approval | 01/11/2021 |
| BUPROPION HYDROCHLORIDE | ANDA | BUPROPION HYDROCHLORIDE | SANDOZ | Labeling | Approval | 01/11/2021 |
| LEVETIRACETAM | ANDA | LEVETIRACETAM | ZYDUS PHARMS USA INC | Labeling | Approval | 01/11/2021 |
| LEVETIRACETAM | ANDA | LEVETIRACETAM | ZYDUS PHARMS USA INC | Labeling | Approval | 01/11/2021 |
| LEVETIRACETAM | ANDA | LEVETIRACETAM | ZYDUS PHARMS USA INC | Labeling | Approval | 01/11/2021 |
| NUZYRA | NDA | OMADACYCLINE TOSYLATE | PARATEK PHARMS INC | Labeling | Approval | 01/11/2021 |
| NUZYRA | NDA | OMADACYCLINE TOSYLATE | PARATEK PHARMS INC | Labeling | Approval | 01/11/2021 |
| TESTOSTERONE | ANDA | TESTOSTERONE | FEMPHARM | Approval | 01/11/2021 | |
| ACYCLOVIR | ANDA | ACYCLOVIR | VISTAPHARM | Approval | 01/11/2021 | |
| DARZALEX FASPRO | BLA | DARATUMUMAB;HYALURONIDASE-FIHJ | JANSSEN BIOTECH | Approval | 01/11/2021 | |
| AYUNA | ANDA | ETHINYL ESTRADIOL; LEVONORGESTREL | AUROBINDO PHARMA LTD | Labeling | Approval | 01/12/2021 |
| AYUNA | ANDA | ETHINYL ESTRADIOL; LEVONORGESTREL | AUROBINDO PHARMA LTD | Labeling | Approval | 01/12/2021 |
| FOSAPREPITANT DIMEGLUMINE | ANDA | FOSAPREPITANT DIMEGLUMINE | AUROBINDO PHARMA LTD | Approval | 01/12/2021 | |
| CELECOXIB | ANDA | CELECOXIB | UNICHEM | Approval | 01/12/2021 | |
| ZYPREXA RELPREVV | NDA | OLANZAPINE PAMOATE | ELI LILLY CO | Labeling | Approval | 01/13/2021 |
| SERTRALINE HYDROCHLORIDE | ANDA | SERTRALINE HYDROCHLORIDE | ACI HEALTHCARE LTD | Labeling | Approval | 01/13/2021 |
| DOXYCYCLINE HYCLATE | ANDA | DOXYCYCLINE HYCLATE | ACTAVIS ELIZABETH | Labeling | Approval | 01/13/2021 |
| EMTRICITABINE AND TENOFOVIR DISOPROXIL FUMARATE | ANDA | EMTRICITABINE; TENOFOVIR DISOPROXIL FUMARATE | STRIDES PHARMA | Approval | 01/13/2021 | |
| BUPRENORPHINE HYDROCHLORIDE AND NALOXONE HYDROCHLORIDE | ANDA | BUPRENORPHINE HYDROCHLORIDE; NALOXONE HYDROCHLORIDE | ACTAVIS ELIZABETH | Labeling | Approval | 01/13/2021 |
| NITHIODOTE | NDA | SODIUM NITRITE; SODIUM THIOSULFATE | HOPE PHARMS | Labeling | Approval | 01/13/2021 |
| DOCETAXEL | NDA | DOCETAXEL | SANDOZ | Labeling | Approval | 01/13/2021 |
| ABACAVIR SULFATE | ANDA | ABACAVIR SULFATE | APOTEX INC | Labeling | Approval | 01/13/2021 |
| ABACAVIR SULFATE | ANDA | ABACAVIR SULFATE | APOTEX INC | Labeling | Approval | 01/13/2021 |
| NORETHINDRONE ACETATE AND ETHINYL ESTRADIOL | ANDA | ETHINYL ESTRADIOL; NORETHINDRONE ACETATE | GLENMARK GENERICS | Labeling | Approval | 01/13/2021 |
| ASHLYNA | ANDA | ETHINYL ESTRADIOL; LEVONORGESTREL | GLENMARK GENERICS | Labeling | Approval | 01/13/2021 |
| LEVONORGESTREL AND ETHINYL ESTRADIOL | ANDA | ETHINYL ESTRADIOL; LEVONORGESTREL | GLENMARK PHARMS LTD | Labeling | Approval | 01/13/2021 |
| LEVONORGESTREL AND ETHINYL ESTRADIOL | ANDA | ETHINYL ESTRADIOL; LEVONORGESTREL | GLENMARK PHARMS LTD | Labeling | Approval | 01/13/2021 |
| SODIUM NITRITE | NDA | SODIUM NITRITE | HOPE PHARMS | Labeling | Approval | 01/13/2021 |
| ETHINYL ESTRADIOL; ETONOGESTREL | ANDA | ETHINYL ESTRADIOL; ETONOGESTREL | TEVA PHARMS USA INC | Approval | 01/13/2021 | |
| EZETIMIBE AND ATORVASTATIN CALCIUM | ANDA | ATORVASTATIN CALCIUM; EZETIMIBE | SCOV3 | Labeling | Approval | 01/13/2021 |
| EZETIMIBE AND ATORVASTATIN CALCIUM | ANDA | ATORVASTATIN CALCIUM; EZETIMIBE | SCOV3 | Labeling | Approval | 01/13/2021 |
| EZETIMIBE AND ATORVASTATIN CALCIUM | ANDA | ATORVASTATIN CALCIUM; EZETIMIBE | SCOV3 | Labeling | Approval | 01/13/2021 |
| TRAMADOL HYDROCHLORIDE | ANDA | TRAMADOL HYDROCHLORIDE | RUBICON | Labeling | Approval | 01/13/2021 |
| TRAMADOL HYDROCHLORIDE | ANDA | TRAMADOL HYDROCHLORIDE | RUBICON | Labeling | Approval | 01/13/2021 |
| DEXTROAMP SACCHARATE, AMP ASPARTATE, DEXTROAMP SULFATE AND AMP SULFATE | ANDA | AMPHETAMINE ASPARTATE ; AMPHETAMINE SULFATE; DEXTROAMPHETAMINE SACCHARATE; DEXTROAMPHETAMINE SULFATE | RHODES PHARMS | Approval | 01/13/2021 | |
| DEXTROAMP SACCHARATE, AMP ASPARTATE, DEXTROAMP SULFATE AND AMP SULFATE | ANDA | AMPHETAMINE ASPARTATE; AMPHETAMINE SULFATE; DEXTROAMPHETAMINE SACCHARATE; DEXTROAMPHETAMINE SULFATE | RHODES PHARMS | Approval | 01/13/2021 | |
| GEMFIBROZIL | ANDA | GEMFIBROZIL | ASCENT PHARMS INC | Approval | 01/13/2021 | |
| VFEND | NDA | VORICONAZOLE | PF PRISM CV | Labeling | Approval | 01/14/2021 |
| VFEND | NDA | VORICONAZOLE | PF PRISM CV | Labeling | Approval | 01/14/2021 |
| VFEND | NDA | VORICONAZOLE | PF PRISM CV | Labeling | Approval | 01/14/2021 |
| AMOXICILLIN | ANDA | AMOXICILLIN | WOCKHARDT BIO AG | Labeling | Approval | 01/14/2021 |
| AMOXICILLIN | ANDA | AMOXICILLIN | WOCKHARDT BIO AG | Labeling | Approval | 01/14/2021 |
| VINCRISTINE SULFATE PFS | ANDA | VINCRISTINE SULFATE | HOSPIRA | Labeling | Approval | 01/14/2021 |
| RASAGILINE MESYLATE | ANDA | RASAGILINE MESYLATE | ALKEM LABS LTD | Labeling | Approval | 01/14/2021 |
| RASAGILINE MESYLATE | ANDA | RASAGILINE MESYLATE | MYLAN | Labeling | Approval | 01/14/2021 |
| XALKORI | NDA | CRIZOTINIB | PF PRISM CV | Efficacy | Approval | 01/14/2021 |
| BUPRENORPHINE HYDROCHLORIDE AND NALOXONE HYDROCHLORIDE | ANDA | BUPRENORPHINE HYDROCHLORIDE; NALOXONE HYDROCHLORIDE | AMNEAL PHARMS | Labeling | Approval | 01/14/2021 |
| BUPRENORPHINE HYDROCHLORIDE AND NALOXONE HYDROCHLORIDE | ANDA | BUPRENORPHINE HYDROCHLORIDE; NALOXONE HYDROCHLORIDE | AMNEAL PHARMS | Labeling | Approval | 01/14/2021 |
| DOCETAXEL | ANDA | DOCETAXEL | HIKMA | Approval | 01/14/2021 | |
| SEVELAMER CARBONATE | ANDA | SEVELAMER CARBONATE | LUPIN LTD | Approval | 01/14/2021 | |
| RASAGILINE MESYLATE | ANDA | RASAGILINE MESYLATE | INDOCO | Labeling | Approval | 01/14/2021 |
| GIMOTI | NDA | METOCLOPRAMIDE HYDROCHLORIDE | EVOKE PHARMA INC | Labeling | Approval | 01/14/2021 |
| BEXAROTENEA | ANDA | BEXAROTENE | TEVA PHARMS USA | Approval | 01/14/2021 | |
| ALBUTEROL SULFATE | ANDA | ALBUTEROL SULFATE | LUPIN | Manufacturing (CMC) | Approval | 01/14/2021 |
| SOLIFENACIN SUCCINATE | ANDA | SOLIFENACIN SUCCINATE | JIANGXI BOYA SEEHOT | Labeling | Approval | 01/14/2021 |
| SOLIFENACIN SUCCINATE | ANDA | SOLIFENACIN SUCCINATE | RISING | Labeling | Approval | 01/14/2021 |
| JELMYTO | NDA | MITOMYCIN | UROGEN PHARMA | Labeling | Approval | 01/14/2021 |
| NALOXONE HYDROCHLORIDE | ANDA | NALOXONE HYDROCHLORIDE | AUROBINDO PHARMA LTD | Approval | 01/14/2021 | |
| METFORMIN HYDROCHLORIDE | ANDA | METFORMIN HYDROCHLORIDE | GRANULES | Approval | 01/14/2021 | |
| NYSTATIN | ANDA | NYSTATIN | MACLEODS PHARMS LTD | Approval | 01/14/2021 | |
| AZULFIDINE | NDA | SULFASALAZINE | PHARMACIA AND UPJOHN | Labeling | Approval | 01/15/2021 |
| AZULFIDINE EN-TABS | NDA | SULFASALAZINE | PHARMACIA AND UPJOHN | Labeling | Approval | 01/15/2021 |
| CELLCEPT | NDA | MYCOPHENOLATE MOFETIL | ROCHE PALO | REMS | Approval | 01/15/2021 |
| CELLCEPT | NDA | MYCOPHENOLATE MOFETIL | ROCHE PALO | REMS | Approval | 01/15/2021 |
| CELLCEPT | NDA | MYCOPHENOLATE MOFETIL HYDROCHLORIDE | ROCHE PALO | REMS | Approval | 01/15/2021 |
| CELLCEPT | NDA | MYCOPHENOLATE MOFETIL | ROCHE PALO | REMS | Approval | 01/15/2021 |
| MYFORTIC | NDA | MYCOPHENOLIC ACID | NOVARTIS | REMS | Approval | 01/15/2021 |
| MYCOPHENOLATE MOFETIL | ANDA | MYCOPHENOLATE MOFETIL | SANDOZ | REMS | Approval | 01/15/2021 |
| MYCOPHENOLATE MOFETIL | ANDA | MYCOPHENOLATE MOFETIL | HIKMA | REMS | Approval | 01/15/2021 |
| MYCOPHENOLATE MOFETIL | ANDA | MYCOPHENOLATE MOFETIL | HIKMA | REMS | Approval | 01/15/2021 |
| MYCOPHENOLATE MOFETIL | ANDA | MYCOPHENOLATE MOFETIL | ACCORD HLTHCARE | REMS | Approval | 01/15/2021 |
| MYCOPHENOLATE MOFETIL | ANDA | MYCOPHENOLATE MOFETIL | SANDOZ | REMS | Approval | 01/15/2021 |
| MYCOPHENOLATE MOFETIL | ANDA | MYCOPHENOLATE MOFETIL | TEVA PHARMS | REMS | Approval | 01/15/2021 |
| MYCOPHENOLATE MOFETIL | ANDA | MYCOPHENOLATE MOFETIL | TEVA PHARMS | REMS | Approval | 01/15/2021 |
| MYCOPHENOLATE MOFETIL | ANDA | MYCOPHENOLATE MOFETIL | MYLAN | REMS | Approval | 01/15/2021 |
| MYCOPHENOLATE MOFETIL | ANDA | MYCOPHENOLATE MOFETIL | MYLAN | REMS | Approval | 01/15/2021 |
| MYCOPHENOLATE MOFETIL | ANDA | MYCOPHENOLATE MOFETIL | STRIDES PHARMA | REMS | Approval | 01/15/2021 |
| MYCOPHENOLATE MOFETIL | ANDA | MYCOPHENOLATE MOFETIL | VINTAGE PHARMS LLC | REMS | Approval | 01/15/2021 |
| MYCOPHENOLATE MOFETIL | ANDA | MYCOPHENOLATE MOFETIL | ACCORD HLTHCARE | REMS | Approval | 01/15/2021 |
| MYCOPHENOLATE MOFETIL | ANDA | MYCOPHENOLATE MOFETIL | STRIDES PHARMA | REMS | Approval | 01/15/2021 |
| MYCOPHENOLATE MOFETIL | ANDA | MYCOPHENOLATE MOFETIL | AMNEAL | REMS | Approval | 01/15/2021 |
| MYCOPHENOLATE MOFETIL | ANDA | MYCOPHENOLATE MOFETIL | JUBILANT CADISTA | REMS | Approval | 01/15/2021 |
| MYCOPHENOLATE MOFETIL | ANDA | MYCOPHENOLATE MOFETIL | JUBILANT CADISTA | REMS | Approval | 01/15/2021 |
| ATORVASTATIN CALCIUM | ANDA | ATORVASTATIN CALCIUM | MYLAN PHARMS INC | Manufacturing (CMC) | Approval | 01/15/2021 |
| ATORVASTATIN CALCIUM | ANDA | ATORVASTATIN CALCIUM | MYLAN PHARMS INC | Labeling | Approval | 01/15/2021 |
| ATORVASTATIN CALCIUM | ANDA | ATORVASTATIN CALCIUM | MYLAN PHARMS INC | Labeling | Approval | 01/15/2021 |
| ATORVASTATIN CALCIUM | ANDA | ATORVASTATIN CALCIUM | MYLAN PHARMS INC | Labeling | Approval | 01/15/2021 |
| ATORVASTATIN CALCIUM | ANDA | ATORVASTATIN CALCIUM | MYLAN PHARMS INC | Labeling | Approval | 01/15/2021 |
| MYCOPHENOLIC ACID | ANDA | MYCOPHENOLIC ACID | RK PHARMA | REMS | Approval | 01/15/2021 |
| MYCOPHENOLATE MOFETIL | ANDA | MYCOPHENOLATE MOFETIL | ALKEM LABS LTD | REMS | Approval | 01/15/2021 |
| MYCOPHENOLIC ACID | ANDA | MYCOPHENOLIC ACID | APOTEX INC | REMS | Approval | 01/15/2021 |
| MYCOPHENOLATE MOFETIL | ANDA | MYCOPHENOLATE MOFETIL | ALKEM LABS LTD | REMS | Approval | 01/15/2021 |
| MYCOPHENOLIC ACID | ANDA | MYCOPHENOLIC ACID | ACCORD HLTHCARE | REMS | Approval | 01/15/2021 |
| MYCOPHENOLIC ACID | ANDA | MYCOPHENOLIC ACID | TEVA PHARMS USA | REMS | Approval | 01/15/2021 |
| MYCOPHENOLATE MOFETIL | ANDA | MYCOPHENOLATE MOFETIL | ALKEM LABS LTD | REMS | Approval | 01/15/2021 |
| MYCOPHENOLATE MOFETIL HYDROCHLORIDE | ANDA | MYCOPHENOLATE MOFETIL HYDROCHLORIDE | PAR STERILE PRODUCTS | REMS | Approval | 01/15/2021 |
| MYCOPHENOLATE MOFETIL HYDROCHLORIDE | ANDA | MYCOPHENOLATE MOFETIL HYDROCHLORIDE | MYLAN LABS LTD | REMS | Approval | 01/15/2021 |
| MYCOPHENOLATE MOFETIL HYDROCHLORIDE | ANDA | MYCOPHENOLATE MOFETIL HYDROCHLORIDE | AKORN INC | REMS | Approval | 01/15/2021 |
| MYCOPHENOLATE MOFETIL | ANDA | MYCOPHENOLATE MOFETIL | ZHEJIANG HISUN PHARM | REMS | Approval | 01/15/2021 |
| MYCOPHENOLATE MOFETIL | ANDA | MYCOPHENOLATE MOFETIL | ZHEJIANG HISUN PHARM | REMS | Approval | 01/15/2021 |
| MYCOPHENOLATE MOFETIL HYDROCHLORIDE | ANDA | MYCOPHENOLATE MOFETIL HYDROCHLORIDE | ZYDUS PHARMS | REMS | Approval | 01/15/2021 |
| EZETIMIBE AND ATORVASTATIN CALCIUM | ANDA | ATORVASTATIN CALCIUM; EZETIMIBE | SCOV3 | Labeling | Approval | 01/15/2021 |
| FERUMOXYTOL | ANDA | FERUMOXYTOL | SANDOZ INC | Approval | 01/15/2021 | |
| ACETAMINOPHE | NDA | ACETAMINOPHEN | MYLAN LABS LTD | Type 5 – New Formulation or New Manufacturer | Approval | 01/15/2021 |
| MYCOPHENOLATE MOFETIL | ANDA | MYCOPHENOLATE MOFETIL | CONCORD BIOTECH LTD | REMS | Approval | 01/15/2021 |
| MYCOPHENOLATE MOFETIL | ANDA | MYCOPHENOLATE MOFETIL | VISTAPHARM | REMS | Approval | 01/15/2021 |
| EPOPROSTENOL SODIUM | ANDA | EPOPROSTENOL SODIUM | SUN PHARM | Approval | 01/15/2021 | |
| BACLOFEN | ANDA | BACLOFEN | MAIA PHARMS INC | Approval | 01/15/2021 | |
| MYCOPHENOLIC ACID | ANDA | MYCOPHENOLIC ACID | CONCORD BIOTECH LTD | REMS | Approval | 01/15/2021 |
| METHYLERGONOVINE MALEATE | ANDA | METHYLERGONOVINE MALEATE | RISING | Approval | 01/15/2021 | |
| MYCOPHENOLATE MOFETIL | ANDA | MYCOPHENOLATE MOFETIL | CONCORD BIOTECH LTD | REMS | Approval | 01/15/2021 |
| MYCOPHENOLATE MOFETIL HYDROCHLORIDE | ANDA | MYCOPHENOLATE MOFETIL HYDROCHLORIDE | NANJING KING-FRIEND | Approval | 01/15/2021 | |
| REGADENOSON | ANDA | REGADENOSON | DR REDDYS LABS LTD | Tentative Approval | 01/15/2021 | |
| NOREPINEPHRINE BITARTRATE IN 5% DEXTROSE | NDA | NOREPINEPHRINE BITARTRATE | BAXTER HLTHCARE CORP | Type 5 – New Formulation or New Manufacturer | Approval | 01/15/2021 |
| ENHERTU | BLA | FAM-TRASTUZUMAB DERUXTECAN-NXKI | DAIICHI SANKYO | Approval | 01/15/2021 | |
| DARZALEX FASPRO | BLA | DARATUMUMAB;HYALURONIDASE-FIHJ | JANSSEN BIOTECH | Approval | 01/15/2021 | |
| FASLODEX | NDA | FULVESTRANT | ASTRAZENECA | Labeling | Approval | 01/19/2021 |
| BROMOCRIPTINE MESYLATE | ANDA | BROMOCRIPTINE MESYLATE | ZYDUS PHARMS USA INC | Labeling | Approval | 01/19/2021 |
| FLUDEOXYGLUCOSE F18 | ANDA | FLUDEOXYGLUCOSE F-18 | WISCONSIN | Labeling | Approval | 01/19/2021 |
| FLUDEOXYGLUCOSE F18 | ANDA | FLUDEOXYGLUCOSE F-18 | BIOMEDCL RES FDN | Labeling | Approval | 01/19/2021 |
| BLOXIVERZ | NDA | NEOSTIGMINE METHYLSULFATE | EXELA PHARMA | Labeling | Approval | 01/19/2021 |
| SPRITAM | NDA | LEVETIRACETAM | APRECIA PHARMS | Efficacy | Approval | 01/19/2021 |
| FINGOLIMOD HYDROCHLORIDE | ANDA | FINGOLIMOD HYDROCHLORIDE | MYLAN | Approval | 01/19/2021 | |
| LAMOTRIGINE | ANDA | LAMOTRIGINE | AJANTA PHARMA LTD | Approval | 01/19/2021 | |
| VERQUVO | NDA | VERICIGUAT | MERCK SHARP DOHME | Type 1 – New Molecular Entity | Approval | 01/19/2021 |
| VENOFER | NDA | IRON SUCROSE | AM REGENT | Labeling | Approval | 01/20/2021 |
| MACRODANTIN | NDA | NITROFURANTOIN, MACROCRYSTALLINE | ALMATICA | Labeling | Approval | 01/21/2021 |
| MACROBID | NDA | NITROFURANTOIN; NITROFURANTOIN, MACROCRYSTALLINE | ALMATICA | Labeling | Approval | 01/21/2021 |
| CLONAZEPAM | ANDA | CLONAZEPAM | SUN PHARM INDS INC | Labeling | Approval | 01/21/2021 |
| PROCTOFOAM HC | ANDA | HYDROCORTISONE ACETATE; PRAMOXINE HYDROCHLORIDE | MYLAN SPECIALITY LP | Manufacturing (CMC) | Approval | 01/21/2021 |
| MEMANTINE HYDROCHLORIDE | ANDA | MEMANTINE HYDROCHLORIDE | ORCHID HLTHCARE | Labeling | Approval | 01/21/2021 |
| FLUOXETINE HYDROCHLORIDE | ANDA | FLUOXETINE HYDROCHLORIDE | ALEMBIC PHARMS LTD | Labeling | Approval | 01/21/2021 |
| FLUOXETINE HYDROCHLORIDE | ANDA | FLUOXETINE HYDROCHLORIDE | ALEMBIC PHARMS LTD | Labeling | Approval | 01/21/2021 |
| EPINASTINE HYDROCHLORIDE | ANDA | EPINASTINE HYDROCHLORIDE | BRECKENRIDGE | Labeling | Approval | 01/21/2021 |
| LEVOFLOXACIN | ANDA | LEVOFLOXACIN | MACLEODS PHARMS LTD | Labeling | Approval | 01/21/2021 |
| LEVOFLOXACIN | ANDA | LEVOFLOXACIN | MACLEODS PHARMS LTD | Labeling | Approval | 01/21/2021 |
| LEVOFLOXACIN | ANDA | LEVOFLOXACIN | MACLEODS PHARMS LTD | Labeling | Approval | 01/21/2021 |
| EDURANT | NDA | RILPIVIRINE HYDROCHLORIDE | JANSSEN PRODS | Efficacy | Approval | 01/21/2021 |
| OLMESARTAN MEDOXOMIL AND HYDROCHLOROTHIAZIDE | ANDA | HYDROCHLOROTHIAZIDE; OLMESARTAN MEDOXOMIL | ALEMBIC PHARMS LTD | Labeling | Approval | 01/21/2021 |
| DOXYCYCLINE | ANDA | DOXYCYCLINE | MYLAN | Approval | 01/21/2021 | |
| OLMESARTAN MEDOXOMIL AND HYDROCHLOROTHIAZIDE | ANDA | HYDROCHLOROTHIAZIDE; OLMESARTAN MEDOXOMIL | ACCORD HLTHCARE | Labeling | Approval | 01/21/2021 |
| OSELTAMIVIR PHOSPHATE | ANDA | OSELTAMIVIR PHOSPHATE | MYLAN | Approval | 01/21/2021 | |
| VOCABRIA | NDA | CABOTEGRAVIR SODIUM | VIIV HLTHCARE | Type 1 – New Molecular Entity | Approval | 01/21/2021 |
| CABENUVA | NDA | CABOTEGRAVIR;RILPIVIRINE | VIIV HLTHCARE | Type 1 – New Molecular Entity and Type 4 – New Combination | Approval | 01/21/2021 |
| VENLAFAXINE HYDROCHLORIDE | ANDA | VENLAFAXINE HYDROCHLORIDE | DEXCEL PHARMA | Approval | 01/21/2021 | |
| ARGATROBAN | ANDA | ARGATROBAN | CAPLIN | Approval | 01/21/2021 | |
| MIDODRINE HYDROCHLORIDE | ANDA | MIDODRINE HYDROCHLORIDE | ALEMBIC PHARMS LTD | Approval | 01/21/2021 | |
| BRIDION | NDA | SUGAMMADEX SODIUM | ORGANON SUB MERCK | Efficacy | Approval | 01/22/2021 |
| BRIDION | NDA | SUGAMMADEX SODIUM | ORGANON SUB MERCK | Labeling | Approval | 01/22/2021 |
| CARBAGLU | NDA | CARGLUMIC ACID | RECORDATI RARE | Efficacy | Approval | 01/22/2021 |
| CARBAGLU | NDA | CARGLUMIC ACID | RECORDATI RARE | Efficacy | Approval | 01/22/2021 |
| CLONAZEPAM | ANDA | CLONAZEPAM | PAR PHARM | Labeling | Approval | 01/22/2021 |
| OPDIVO | BLA | NIVOLUMAB | BRISTOL MYERS SQUIBB | Approval | 01/22/2021 | |
| FLUDEOXYGLUCOSE F18 | ANDA | FLUDEOXYGLUCOSE F-18 | BIOMEDCL RES FDN | Labeling | Approval | 01/22/2021 |
| TRINTELLIX | NDA | VORTIOXETINE HYDROBROMIDE | TAKEDA PHARMS USA | Efficacy | Approval | 01/22/2021 |
| TRINTELLIX | NDA | VORTIOXETINE HYDROBROMIDE | TAKEDA PHARMS USA | Labeling | Approval | 01/22/2021 |
| CABOMETYX | NDA | CABOZANTINIB S-MALATE | EXELIXIS INC | Efficacy | Approval | 01/22/2021 |
| PIOGLITAZONE HYDROCHLORIDE | ANDA | PIOGLITAZONE HYDROCHLORIDE | SHUANGCHENG | Approval | 01/22/2021 | |
| URSODIOL | ANDA | URSODIOL | STRIDES PHARMA | Approval | 01/22/2021 | |
| LORBRENA | NDA | LORLATINIB | PFIZER | Labeling | Approval | 01/22/2021 |
| TOBRAMYCIN | ANDA | TOBRAMYCIN | AUROBINDO PHARMA LTD | Approval | 01/22/2021 | |
| CHLORPROMAZINE HYDROCHLORIDE | ANDA | CHLORPROMAZINE HYDROCHLORIDE | LANNETT CO INC | Approval | 01/22/2021 | |
| ALBENDAZOLE | NDA | ALBENDAZOLE | MSN | Approval | 01/22/2021 | |
| AMPHETAMINE SULFATE | ANDA | AMPHETAMINE SULFATE | SPECGX LLC | Approval | 01/22/2021 | |
| LUPKYNIS | NDA | VOCLOSPORIN | AURINIA PHARMACEUTICALS, INC. | Type 1 – New Molecular Entity | Approval | 01/22/2021 |
| NOREPINEPHRINE BITARTRATE | ANDA | NOREPINEPHRINE BITARTRATE | ANDERSEN GLOBAL | Approval | 01/22/2021 | |
| LIOTHYRONINE SODIUM | ANDA | LIOTHYRONINE SODIUM | ZYDUS | Approval | 01/22/2021 | |
| SILIQ | BLA | BRODALUMAB | VALEANT LUXEMBOURG | Approval | 01/22/2021 | |
| MAYZENT | NDA | SIPONIMOD FUMARIC ACID | NOVARTIS | Labeling | Approval | 01/23/2021 |
| VFEND | NDA | VORICONAZOLE | PF PRISM CV | Labeling | Approval | 01/25/2021 |
| VFEND | NDA | VORICONAZOLE | PF PRISM CV | Labeling | Approval | 01/25/2021 |
| VFEND | NDA | VORICONAZOLE | PF PRISM CV | Labeling | Approval | 01/25/2021 |
| MERCAPTOPURINE | ANDA | MERCAPTOPURINE | HIKMA | Labeling | Approval | 01/25/2021 |
| MERCAPTOPURINE | ANDA | MERCAPTOPURINE | HIKMA | Labeling | Approval | 01/25/2021 |
| CLONAZEPAM | ANDA | CLONAZEPAM | BARR | Labeling | Approval | 01/25/2021 |
| OLMESARTAN MEDOXOMIL AND HYDROCHLOROTHIAZIDE | ANDA | HYDROCHLOROTHIAZIDE; OLMESARTAN MEDOXOMIL | MYLAN | Labeling | Approval | 01/25/2021 |
| MEMANTINE HYDROCHLORIDE | ANDA | MEMANTINE HYDROCHLORIDE | CELLTRION | Labeling | Approval | 01/25/2021 |
| TIGECYCLINE | ANDA | TIGECYCLINE | SANDOZ INC | Labeling | Approval | 01/25/2021 |
| TRANEXAMIC ACID | ANDA | TRANEXAMIC ACID | AM REGENT | Labeling | Approval | 01/25/2021 |
| MONTELUKAST SODIUM | ANDA | MONTELUKAST SODIUM | ACCORD HLTHCARE | Labeling | Approval | 01/25/2021 |
| OLMESARTAN MEDOXOMIL AND HYDROCHLOROTHIAZIDE | ANDA | HYDROCHLOROTHIAZIDE; OLMESARTAN MEDOXOMIL | AUROBINDO PHARMA LTD | Labeling | Approval | 01/25/2021 |
| MEMANTINE HYDROCHLORIDE | ANDA | MEMANTINE HYDROCHLORIDE | AJANTA PHARMA LTD | Labeling | Approval | 01/25/2021 |
| OLMESARTAN MEDOXOMIL AND HYDROCHLOROTHIAZIDE | ANDA | HYDROCHLOROTHIAZIDE; OLMESARTAN MEDOXOMIL | UMEDICA LABS PVT LTD | Labeling | Approval | 01/25/2021 |
| DOXYCYCLINE | ANDA | DOXYCYCLINE | ALEMBIC PHARMS LTD | Labeling | Approval | 01/25/2021 |
| DOXYCYCLINE | ANDA | DOXYCYCLINE | ALEMBIC PHARMS LTD | Labeling | Approval | 01/25/2021 |
| SELENIOUS ACID | NDA | SELENIOUS ACID | AM REGENT | Manufacturing (CMC) | Approval | 01/25/2021 |
| DOXYCYCLINE | ANDA | DOXYCYCLINE | MAYNE PHARMA INC | Labeling | Approval | 01/25/2021 |
| OLOPATADINE HYDROCHLORIDE | ANDA | OLOPATADINE HYDROCHLORIDE | ALEMBIC PHARMS LTD | Labeling | Approval | 01/25/2021 |
| ARSENIC TRIOXIDE | ANDA | ARSENIC TRIOXIDE | AMNEAL | Approval | 01/25/2021 | |
| MACITENTAN | ANDA | MACITENTAN | SUN PHARM INDS | Tentative Approval | 01/25/2021 | |
| SOLIFENACIN SUCCINATE | ANDA | SOLIFENACIN SUCCINATE | UNICHEM | Labeling | Approval | 01/25/2021 |
| DECITABINE | ANDA | DECITABINE | MSN | Labeling | Approval | 01/25/2021 |
| MEXILETINE HYDROCHLORIDE | ANDA | MEXILETINE HYDROCHLORIDE | RICONPHARMA LLC | Approval | 01/25/2021 | |
| DELFLEX W/ DEXTROSE 1.5% IN PLASTIC CONTAINER | NDA | CALCIUM CHLORIDE; DEXTROSE; MAGNESIUM CHLORIDE; SODIUM CHLORIDE; SODIUM LACTATE | FRESENIUS MEDCL | Labeling | Approval | 01/26/2021 |
| DELFLEX W/ DEXTROSE 1.5% LOW MAGNESIUM IN PLASTIC CONTAINER | NDA | CALCIUM CHLORIDE; DEXTROSE; MAGNESIUM CHLORIDE; SODIUM CHLORIDE; SODIUM LACTATE | FRESENIUS MEDCL | Labeling | Approval | 01/26/2021 |
| DELFLEX W/ DEXTROSE 2.5% IN PLASTIC CONTAINER | NDA | CALCIUM CHLORIDE; DEXTROSE; MAGNESIUM CHLORIDE; SODIUM CHLORIDE; SODIUM LACTATE | FRESENIUS MEDCL | Labeling | Approval | 01/26/2021 |
| DELFLEX W/ DEXTROSE 2.5% LOW MAGNESIUM IN PLASTIC CONTAINER | NDA | CALCIUM CHLORIDE; DEXTROSE; MAGNESIUM CHLORIDE; SODIUM CHLORIDE; SODIUM LACTATE | FRESENIUS MEDCL | Labeling | Approval | 01/26/2021 |
| DELFLEX W/ DEXTROSE 4.25% IN PLASTIC CONTAINER | NDA | CALCIUM CHLORIDE; DEXTROSE; MAGNESIUM CHLORIDE; SODIUM CHLORIDE; SODIUM LACTATE | FRESENIUS MEDCL | Labeling | Approval | 01/26/2021 |
| DELFLEX W/ DEXTROSE 4.25% LOW MAGNESIUM IN PLASTIC CONTAINER | NDA | CALCIUM CHLORIDE; DEXTROSE; MAGNESIUM CHLORIDE; SODIUM CHLORIDE; SODIUM LACTATE | FRESENIUS MEDCL | Labeling | Approval | 01/26/2021 |
| DELFLEX W/ DEXTROSE 1.5% LOW MAGNESIUM LOW CALCIUM IN PLASTIC CONTAINER | NDA | CALCIUM CHLORIDE; DEXTROSE; MAGNESIUM CHLORIDE; SODIUM CHLORIDE; SODIUM LACTATE | FRESENIUS MEDCL | Labeling | Approval | 01/26/2021 |
| DELFLEX W/ DEXTROSE 2.5% LOW MAGNESIUM LOW CALCIUM IN PLASTIC CONTAINER | NDA | CALCIUM CHLORIDE; DEXTROSE; MAGNESIUM CHLORIDE; SODIUM CHLORIDE; SODIUM LACTATE | FRESENIUS MEDCL | Labeling | Approval | 01/26/2021 |
| DELFLEX W/ DEXTROSE 4.25% LOW MAGNESIUM LOW CALCIUM IN PLASTIC CONTAINER | NDA | CALCIUM CHLORIDE; DEXTROSE; MAGNESIUM CHLORIDE; SODIUM CHLORIDE; SODIUM LACTATE | FRESENIUS MEDCL | Labeling | Approval | 01/26/2021 |
| GLUCAGON | NDA | GLUCAGON | LILLY | Labeling | Approval | 01/26/2021 |
| LAMIVUDINE | ANDA | LAMIVUDINE | APOTEX | Labeling | Approval | 01/26/2021 |
| LAMIVUDINE | ANDA | LAMIVUDINE | APOTEX | Labeling | Approval | 01/26/2021 |
| LAMIVUDINE | ANDA | LAMIVUDINE | APOTEX | Labeling | Approval | 01/26/2021 |
| BENDAMUSTINE HYDROCHLORIDE | NDA | BENDAMUSTINE HYDROCHLORIDE | APOTEX INC | Tentative Approval | 01/26/2021 | |
| IMIQUIMOD | ANDA | IMIQUIMOD | TARO | Approval | 01/26/2021 | |
| VIGABATRIN | ANDA | VIGABATRIN | TEVA PHARMS USA | Labeling | Approval | 01/26/2021 |
| VIGABATRIN | ANDA | VIGABATRIN | TEVA PHARMS USA | Labeling | Approval | 01/26/2021 |
| ALBENDAZOLE | ANDA | ALBENDAZOLE | DR REDDYS | Approval | 01/26/2021 | |
| TADALAFIL | ANDA | TADALAFIL | TORRENT | Labeling | Approval | 01/26/2021 |
| VIGABATRIN | ANDA | VIGABATRIN | ANNORA PHARMA | Approval | 01/26/2021 | |
| AMINOCAPROIC ACID | ANDA | AMINOCAPROIC ACID | LEADING PHARMA LLC | Approval | 01/26/2021 | |
| ZONISAMIDE | ANDA | ZONISAMIDE | UNICHEM | Approval | 01/26/2021 | |
| DIPYRIDAMOLE | ANDA | DIPYRIDAMOLE | ZYDUS PHARMS USA INC | Labeling | Approval | 01/27/2021 |
| DIPYRIDAMOLE | ANDA | DIPYRIDAMOLE | ZYDUS PHARMS USA INC | Labeling | Approval | 01/27/2021 |
| SOTALOL HYDROCHLORIDE | ANDA | SOTALOL HYDROCHLORIDE | APOTEX | Labeling | Approval | 01/27/2021 |
| SOTALOL HYDROCHLORIDE | ANDA | SOTALOL HYDROCHLORIDE | APOTEX | Labeling | Approval | 01/27/2021 |
| NAFCILLIN SODIUM | ANDA | NAFCILLIN SODIUM | ANTIBIOTICE | Labeling | Approval | 01/27/2021 |
| NAFCILLIN SODIUM | ANDA | NAFCILLIN SODIUM | ANTIBIOTICE | Labeling | Approval | 01/27/2021 |
| NAFCILLIN SODIUM | ANDA | NAFCILLIN SODIUM | SAGENT PHARMS | Labeling | Approval | 01/27/2021 |
| NAFCILLIN SODIUM | ANDA | NAFCILLIN SODIUM | SAGENT PHARMS | Labeling | Approval | 01/27/2021 |
| NAFCILLIN SODIUM | ANDA | NAFCILLIN SODIUM | SAGENT PHARMS | Labeling | Approval | 01/27/2021 |
| NAFCILLIN SODIUM | ANDA | NAFCILLIN SODIUM | SAGENT PHARMS | Labeling | Approval | 01/27/2021 |
| FLUDEOXYGLUCOSE F18 | ANDA | FLUDEOXYGLUCOSE F-18 | JUBILANT DRAXIMAGE | Labeling | Approval | 01/27/2021 |
| DIPHENOXYLATE HYDROCHLORIDE AND ATROPINE SULFATE | ANDA | ATROPINE SULFATE; DIPHENOXYLATE HYDROCHLORIDE | WINDER LABS LLC | Approval | 01/27/2021 | |
| AMPHETAMINE SULFATE | ANDA | AMPHETAMINE SULFATE | GLENMARK PHARMS LTD | Approval | 01/27/2021 | |
| AMPHETAMINE SULFATE | ANDA | AMPHETAMINE SULFATE | SUN PHARM INDS INC | Approval | 01/27/2021 | |
| FUROSEMIDE | ANDA | FUROSEMIDE | ATHENEX INC | Approval | 01/27/2021 | |
| XARELTO | NDA | RIVAROXABAN | JANSSEN PHARMS | Labeling | Approval | 01/28/2021 |
| NPLATE | BLA | ROMIPLOSTIM | AMGEN | Approval | 01/28/2021 | |
| XARELTO | NDA | RIVAROXABAN | JANSSEN PHARMS | Labeling | Approval | 01/28/2021 |
| RAPIVAB | NDA | PERAMIVIR | BIOCRYST | Efficacy | Approval | 01/28/2021 |
| DICLOFENAC SODIUM | ANDA | DICLOFENAC SODIUM | AKORN | Approval | 01/28/2021 | |
| MODAFINIL | ANDA | MODAFINIL | APPCO | Labeling | Approval | 01/28/2021 |
| EMPAGLIFLOZIN | ANDA | EMPAGLIFLOZIN | ANNORA PHARMA | Tentative Approval | 01/28/2021 | |
| RANOLAZINE | ANDA | RANOLAZINE | GRAVITI PHARMS | Approval | 01/28/2021 | |
| RETEVMO | NDA | SELPERCATINIB | LOXO ONCOLOGY INC | Labeling | Approval | 01/28/2021 |
| POTASSIUM CHLORIDE | ANDA | POTASSIUM CHLORIDE | AMTA | Approval | 01/28/2021 | |
| MERCAPTOPURINE | ANDA | MERCAPTOPURINE | MYLAN | Labeling | Approval | 01/29/2021 |
| VANCOCIN HYDROCHLORIDE | ANDA | VANCOMYCIN HYDROCHLORIDE | ANI PHARMS INC | Labeling | Approval | 01/29/2021 |
| VANCOCIN HYDROCHLORIDE IN PLASTIC CONTAINER+B384 | ANDA | VANCOMYCIN HYDROCHLORIDE | BAXTER HLTHCARE | Labeling | Approval | 01/29/2021 |
| VANCOCIN HYDROCHLORIDE | ANDA | VANCOMYCIN HYDROCHLORIDE | ANI PHARMS INC | Labeling | Approval | 01/29/2021 |
| VANCOCIN HYDROCHLORIDE | ANDA | VANCOMYCIN HYDROCHLORIDE | ANI PHARMS INC | Labeling | Approval | 01/29/2021 |
| VANCOCIN HYDROCHLORIDE | ANDA | VANCOMYCIN HYDROCHLORIDE | ANI PHARMS INC | Labeling | Approval | 01/29/2021 |
| NPLATE | BLA | ROMIPLOSTIM | AMGEN | Approval | 01/29/2021 | |
| PLEGRIDY | BLA | PEGINTERFERON BETA-1A | BIOGEN IDEC INC | Approval | 01/29/2021 | |
| LEVETIRACETAM | ANDA | LEVETIRACETAM | MYLAN PHARMS INC | Labeling | Approval | 01/29/2021 |
| LEVETIRACETAM | ANDA | LEVETIRACETAM | MYLAN PHARMS INC | Labeling | Approval | 01/29/2021 |
| LEVETIRACETAM | ANDA | LEVETIRACETAM | MYLAN PHARMS INC | Labeling | Approval | 01/29/2021 |
| LEVETIRACETAM | ANDA | LEVETIRACETAM | MYLAN PHARMS INC | Labeling | Approval | 01/29/2021 |
| MONTELUKAST SODIUM | ANDA | MONTELUKAST SODIUM | HETERO LABS LTD V | Labeling | Approval | 01/29/2021 |
| GATTEX KIT | NDA | TEDUGLUTIDE RECOMBINANT | NPS PHARMS INC | Labeling | Approval | 01/29/2021 |
| TECFIDER | ANDA | DIMETHYL FUMARATE | BIOGEN IDEC INC | Labeling | Approval | 01/29/2021 |
| METHYLPHENIDATE HYDROCHLORIDE | ANDA | METHYLPHENIDATE HYDROCHLORIDE | ASCENT PHARMS INC | Approval | 01/29/2021 | |
| ALECENS | ANDA | ALECTINIB HYDROCHLORIDE | HOFFMANN-LA ROCHE | Labeling | Approval | 01/29/2021 |
| FIRVANQ KIT | NDA | VANCOMYCIN HYDROCHLORIDE | AZURITY | Labeling | Approval | 01/29/2021 |
| VANCOMYCIN HYDROCHLORIDE | NDA | VANCOMYCIN HYDROCHLORIDE | MYLAN LABS LTD | Labeling | Approval | 01/29/2021 |
| PRAVASTATIN SODIUM | ANDA | PRAVASTATIN SODIUM | BIOCON PHARMA | Labeling | Approval | 01/29/2021 |
| TADALAFIL | ANDA | TADALAFIL | LUPIN LTD | Labeling | Approval | 01/29/2021 |
| VIGABATRIN | ANDA | VIGABATRIN | DR REDDYS | Approval | 01/29/2021 | |
| VUMERITY | NDA | DIROXIMEL FUMARATE | BIOGEN | Labeling | Approval | 01/29/2021 |
| VANCOMYCIN HYDROCHLORIDE | NDA | VANCOMYCIN HYDROCHLORIDE | XELLIA PHARMS APS | Labeling | Approval | 01/29/2021 |
| NOREPINEPHRINE BITARTRATE | ANDA | NOREPINEPHRINE BITARTRATE | SUN PHARM | Approval | 01/29/2021 | |
| AMZEEQ | NDA | MINOCYCLINE HYDROCHLORIDE | FOAMIX | Labeling | Approval | 01/29/2021 |
| FOSCARNET SODIUM | ANDA | FOSCARNET SODIUM | FRESENIUS KABI USA | Approval | 01/29/2021 | |
| POTASSIUM CHLORIDE | ANDA | POTASSIUM CHLORIDE | GRANULES PHARMS | Approval | 01/29/2021 | |
| NALOXONE HYDROCHLORIDE | ANDA | NALOXONE HYDROCHLORIDE | BAXTER HLTHCARE CORP | Approval | 01/29/2021 | |
| UPTRAVI | NDA | SELEXIPAG | ACTELION PHARMS LTD | Efficacy | Approval | 01/30/2021 |
FDA-Approved Drugs@December 2020