Pfizer-BioNTech COVID-19 Vaccine Safety Data

Pfizer-BioNTech COVID-19 Vaccine Safety Data
Pfizer-BioNTech COVID-19 Vaccine Safety Data: The World Health Organization (WHO) declared coronavirus disease 2019 (COVID-19) is a pandemic in March 2020.
COVID-19 is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Pfizer, in collaboration with BioNTech, is developing a vaccine to prevent COVID-19 which is based on the SARS-CoV-2 spike glycoprotein (S) antigen encoded by RNA and formulated in lipid nanoparticles (LNP).
Fact Sheet: Pfizer-BioNTech COVID-19 Vaccine
- The vaccine is a white to off-white, sterile, preservative-free, frozen suspension.
- The vaccine contains a nucleoside-modified messenger RNA (modRNA) encoding the viral spike glycoprotein (S) of SARS-CoV-2.
- Vaccine also contains ingredients: lipids ((4-hydroxybutyl)azanediyl)bis(hexane-6,1-diyl)bis(2- hexyldecanoate), 2-[(polyethylene glycol)-2000]-N,N-ditetradecylacetamide, 1,2-distearoyl-snglycero-3-phosphocholine, and cholesterol), potassium chloride, monobasic potassium phosphate, sodium chloride, dibasic sodium phosphate dihydrate, and sucrose.
- The Pfizer Vaccine is supplied as a frozen [between -80°C to -60°C (- 112°F to -76°F)] multi-dose (5-dose) vial.
- The vaccine must be thawed and diluted in its original vial with 1.8 mL of sterile 0.9% Sodium Chloride Injection, USP prior to administration.
- After dilution, the vial contains 5 doses of 0.3 mL per dose.
- After dilution, the multiple-dose vials must be stored between 2°C to 25°C (35°F to 77°F) and used within 6 hours from the time of dilution.
- Vaccine dose is 30 μg, is administered intramuscularly (IM) as a series of two 30 μg doses (0.3 mL each) 21 days apart.
Pfizer-BioNTech COVID-19 Vaccine Safety Data: Safety Highlights from FDA briefing document
On 20 November 2020, Pfizer and BioNTech submitted an Emergency Use Authorization (EUA) request to FDA for an investigational COVID-19 vaccine (BNT162b2) intended to prevent COVID-19 caused by SARS-CoV-2.
The proposed use under a EUA is for active immunization to prevent COVID-19 caused by SARS-CoV-2 in people 16 years of age and older.
The proposed dosing schedule is 2 doses of 30 µg each, given at 21-day intervals.
The EUA application includes data on safety and efficacy from an ongoing phase 3 randomized, double-blinded, and placebo-controlled trial of BNT162b2 in approximately 44,000 participants.
Data from two ongoing clinical studies were included in the EUA request.
| Study Number | Country | Study description | Study Status |
| C4591001 | USA, Argentina, Brazil, Germany, S. Africa, Turkey | Phase 1,2,3 randomized, placebo-controlled, observerblind; to evaluate safety, immunogenicity, and efficacy of COVID-19 vaccine | Ongoing |
| BNT162-01 | Germany | Phase 1/2 randomized, open label; to evaluate safety and immunogenicity, dose-escalation | Ongoing |
The primary efficacy endpoint is the incidence of COVID-19 in participants without evidence of SARS-CoV-2 infection before or during the 2-dose vaccination regimen.
Safety data from approximately 38,000 participants ≥16 years of age randomized 1:1 to vaccine or placebo with a median of 2 months of follow up after the second dose suggest a favorable safety profile.
There were no specific safety concerns identified that would preclude the issuance of a EUA.
Available safety data from all participants enrolled was consistent with the safety profile for the approximately 38,000 participants with a median follow-up of 2 months and also did not raise specific safety concerns.
The most common solicited adverse reactions were
-
- Injection site reactions (84.1%)
- Fatigue (62.9%)
- Headache (55.1%)
- Muscle pain (38.3%)
- Chills (31.9%)
- Joint pain (23.6%)
- Fever (14.2%)
Non-serious unsolicited adverse events: there were four cases of Bell’s palsy in the vaccine group compared with no cases in the placebo group, though the four cases in the vaccine group do not represent a frequency above that expected in the general population.
There were no notable patterns or numerical imbalances between treatment groups for specific categories of non-serious adverse events (including other neurologic, neuroinflammatory, and thrombotic events) that would suggest a causal relationship to the BNT162b2 vaccine.
Serious Adverse Events (SAE) occurred in 0.0% to 4.6% of participants
- A total of six (2 vaccine, 4 placebo) of 43,448 enrolled participants (0.01%) died during the reporting period
- Both vaccine recipients were >55 years of age
- One experienced a cardiac arrest 62 days after vaccination #2 and died 3 days later
- Other died from arteriosclerosis 3 days after vaccination #1.
- The placebo recipients died from myocardial infarction (n=1), hemorrhagic stroke (n=1), or unknown causes (n=2), and three of the four deaths occurred in the older group (>55 years of age).
- All deaths represent events that occur in the general population of the age groups where they occurred, at a similar rate.
The most common SAEs in the vaccine group which were numerically higher than in the placebo group were
- Appendicitis (0.04%)
- Acute myocardial infarction (0.02%)
- Cerebrovascular accident (0.02%)
The most common SAEs in the placebo arm numerically higher than in the vaccine arm were
- Pneumonia (0.03%)
- Atrial fibrillation (0.02%)
- Syncope (0.02%)
Severe adverse reactions more frequent after the second dose than after Dose 1
Severe adverse reactions less frequent in participants ≥55 years of age (≤ 2.8%) as compared to younger participants (≤4.6%)
Frequency of serious adverse events was low
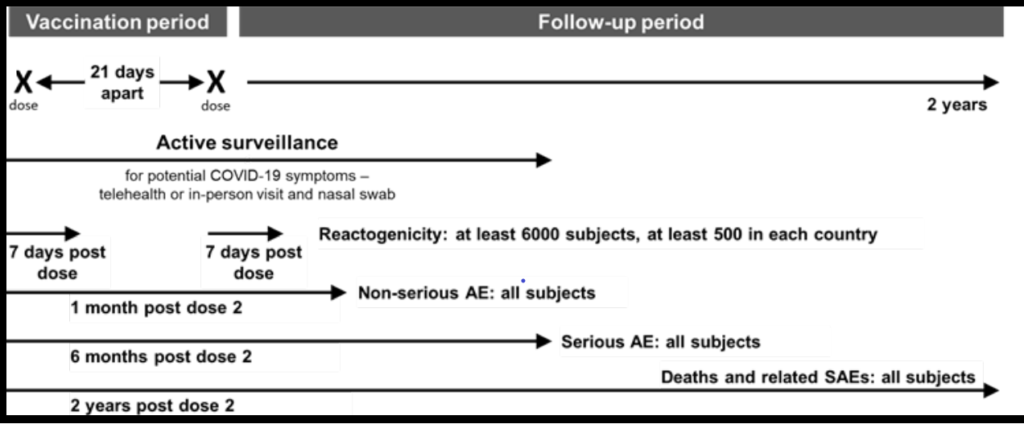
Pharmacovigilance Activities to monitor the safety of COVID-19 vaccine (BNT162b2)
Pfizer submitted a Pharmacovigilance Plan (PVP) to monitor safety concerns
Important potential risk: Vaccine-associated enhanced disease including vaccine-associated enhanced respiratory disease
Missing information: Use in pregnancy and lactation and vaccine effectiveness
Additionally, the FDA requested that the Sponsor update their PVP to include missing information in pediatric participants less than 16 years of age.
The Sponsor will conduct both passive and active surveillance activities for continued vaccine safety monitoring.
Passive surveillance activities will include submitting spontaneous reports of the following events to the Vaccine Adverse Event Reporting System (VAERS) within 15 days:
- Vaccine administration errors whether or not associated with an adverse event
- Serious adverse events (irrespective of attribution to vaccination)
- Cases of Multisystem Inflammatory Syndrome in children and adults
- Cases of COVID-19 that result in hospitalization or death
The Sponsor will also conduct periodic aggregate review of safety data and submit periodic safety reports at monthly intervals.
Each periodic safety report is required to contain descriptive information which includes:
- A narrative summary and analysis of adverse events submitted during the reporting interval, including interval and cumulative counts by age groups, special populations (e.g., pregnant women), and adverse events of special interest
- Newly identified safety concerns in the interval
- Actions taken since the last report because of adverse experiences (e.g., changes made to Vaccination Provider fact sheets, changes made to studies or studies initiated)
Proposed active surveillance studies (three studies) designed to monitor vaccination during pregnancy within populations expected to receive the vaccine under EUA.
Source: Pfizer-BioNTech COVID-19 Vaccine Safety Data: For Full Details: Vaccines and Related Biological Products Advisory Committee Meeting December 10, 2020
One thought on “Pfizer-BioNTech COVID-19 Vaccine Safety Data”