FDA briefed Moderna COVID-19 Vaccine Safety data
FDA briefed Moderna COVID-19 Vaccine Safety data
FDA briefed Moderna COVID-19 Vaccine Safety data
FDA Issued Emergency Use Authorization (EUA) for emergency use of Moderna COVID-19 Vaccine for the prevention of COVID-19 for persons 18 years of age and older
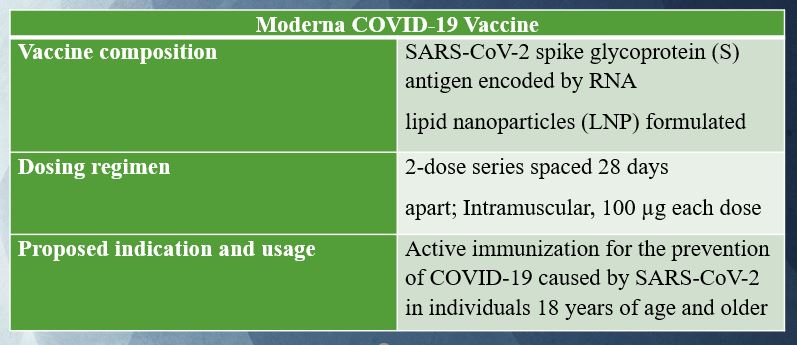
Moderna COVID‑19 Vaccine contains a nucleoside-modified messenger RNA encoding the viral spike (S) glycoprotein of SARS-CoV-2 formulated in lipid particles.
The Moderna Vaccine is supplied as a frozen suspension in multiple-dose vials.
The Moderna Vaccine does not contain a preservative.
Each 0.5 mL dose of the Moderna Vaccine contains 100 mcg of a nucleoside modified messenger RNA encoding the viral spike (S) glycoprotein of SARS-CoV-2.
Each dose of the Moderna Vaccine also includes the following ingredients: lipids (SM-102; 1,2- dimyristoyl-rac-glycero-3-methoxypolyethylene glycol-2000 [PEG2000-DMG]; cholesterol; and 1,2-distearoyl-sn-glycero-3-phosphocholine [DSPC]), tromethamine, tromethamine hydrochloride, acetic acid, sodium acetate, and sucrose.
The dosing regimen is two doses of 0.5 mL each, one month apart.
FDA reviewed the safety and efficacy data from an ongoing study
Clinical Development
| Study
Number |
Study name | Participants | Test Product(s); Dosing Regimens
And Dose levels |
| DMID 20-0003 | Ongoing Phase 1, open-label, doseranging, safety and immunogenicity study in individuals ≥18 years of age | Total of 120 participants in 3 age cohorts:
18-55 yrs (n=60) 56-70 yrs (n=30) ≥71 yrs (n=30) |
mRNA-1273
25 µg 50 µg 100 µg 250 µg |
| P 201 | Ongoing Phase 2 randomized, placebocontrolled, observerblind, dose confirmation study in individuals ≥18 years of age | Total 600 participants 18-54 years (n=300) ≥55 years (n=300) | mRNA-127350 µg
100 µg
|
| P 301 | Ongoing Phase 3, randomized, placebocontrolled, observerblind, efficacy study in individuals ≥18 years of age | 30,351 adults ≥18 years of age | mRNA-1273
2 doses of vaccine (100 µg) |
FDA’s analyzed efficacy data from 28,207 participants 18 years of age and older
The Moderna vaccine has been shown to be approximately 94.5 percent effective in preventing COVID-19.
FDA’s reviewed safety data from 30,351 participants 18 years of age and older
The proportions of participants with SAEs, fatalities, and withdrawals from adverse events were balanced in all study groups.
Solicited local adverse reactions were reported by most vaccinated and at higher rates than placebos.
Vaccine recipients reported higher rates of local reactions following dose 1 than dose 2
The most frequently reported local adverse reactions was injection site pain
The second most frequently reported local adverse reaction was Axillary lymphadenopathy (vaccination arm)
Solicited systemic adverse reactions were reported for the majority of vaccine recipients and at elevated rates compared to placebo recipients.
Vaccine recipients reported higher rates of systemic reactions after the second dose than the first dose.
The most frequently reported systemic adverse reaction was fatigue
Other solicited systemic adverse reactions were headache, myalgia, arthralgia, and chills
Fever was reported following any dose by 14.8% of vaccine participants and 0.6% of placebo beneficiaries.
The frequency of serious adverse events was low (1.0% in the mRNA-1273 arm and 1.0% in the placebo arm), without meaningful imbalances between study arms.
The most common SAEs in the vaccine group which was numerically higher than the placebo group were myocardial infarction, cholecystitis, and nephrolithiasis, although the small numbers of cases of these events do not suggest a causal relationship.
| Adverse reactions | Vaccine Group | Placebo Group |
| Hypersensitivity-related events | 1.5% (n=233) | 1.1% (n=166) |
| Lymphadenopathy-related events | 1.1% (n=173) | 0.63% (n=95) |
| Delayed localized reactions with onset after 7 days seen mostly after dose 1 | 1.4% | 0.4% |
| Bell’s palsy | n=3 | n=1 |
| Serious Adverse Events | ||
| Deaths* (refer FDA briefing document) | 6 | 7 |
Table of Contents
FDA briefed Moderna COVID-19 Vaccine Safety data:
Pharmacovigilance Activities
The Sponsor will conduct both passive and active surveillance activities for continued vaccine safety monitoring.
Passive surveillance activities:
ICSR Reports:
Submitting spontaneous reports (ICSR) of the following events to the Vaccine Adverse Event Reporting System (VAERS):
- Vaccine administration errors whether or not associated with an adverse event
- Serious adverse events (irrespective of attribution to vaccination)
- Cases of Multisystem Inflammatory Syndrome in adults
- Cases of COVID-19 that result in hospitalization or death
These reports should be submitted to VAERS within 15 calendar days from initial receipt of the information by the Company
Aggregate reports:
FDA has requested sponsor that periodic reports be submitted monthly.
- within 15 days after the last day of a month
- beginning after the first full calendar month after the authorization
Each periodic safety report should contain descriptive information which includes:
- A narrative summary and analysis of adverse events submitted during the reporting interval, including interval and cumulative counts by age groups, special populations (e.g., pregnant women), and adverse events of special interest
- Newly identified safety concerns in the interval
- Actions taken since the last report because of adverse experiences (e.g., changes made to Vaccination Provider fact sheets, changes made to studies or studies initiated)
Pharmacovigilance Plan
Safety specifications of the pharmacovigilance plan:
| Important potential risks | Vaccine-associated enhanced disease |
| Anaphylactic reactions (including anaphylaxis) | |
| Important missing information | Use in pregnant and breast-feeding women |
| Use in the pediatric population | |
| Long-term safety and effectiveness | |
| Immunogenicity in subjects with immunosuppression | |
| Concomitant administration with non-COVID vaccines |
PASS Studies
Moderna will conduct post-authorization observational studies to evaluate the association between Moderna COVID-19 Vaccine and a pre-specified list of adverse events of special interest, along with deaths and hospitalizations, and severe COVID-19.
FDA briefed Moderna COVID-19 Vaccine Safety data: Safety Surveillance Studies
Pregnancy Cohort:
Passive pregnancy registry to monitor vaccination during pregnancy within populations expected to receive the vaccine under EUA.
Active Follow-up for Safety:
- Retrospective analyses of medical and pharmacy claims data
- Objectives; estimation of background rates of 23 prespecified AESI, descriptive analyses of observed vs expected rates
- Self-controlled risk interval analyses if certain criteria are met from descriptive analyses