Valid ICSR?

Valid ICSR?
Valid ICSR (Case) is called Potential ICSR (Individual case safety report).
Only valid cases qualify for regulatory submission.
Once receive a case or Adverse event report from any source (Solicited or unsolicited), Drug safety associate performs validity assessment of the report.
Adverse event reports receive from different sources such as
- Clinical trial
- Spontaneous reports
- Literature reports
- License Partners
- Legal cases
- Internet and other media sources
Table of Contents
What is Valid ICSR or Potential ICSR?
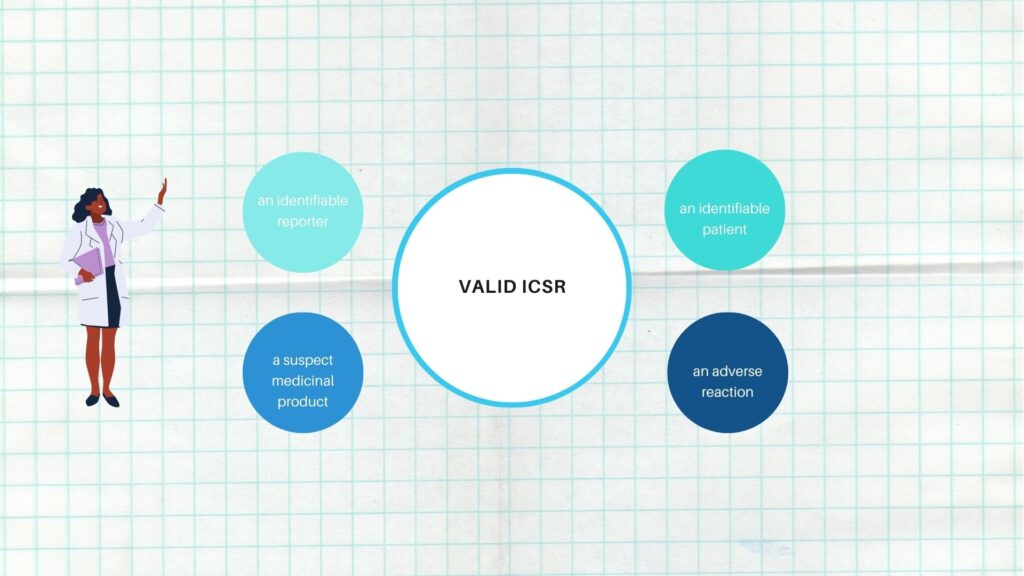
A valid ICSR or Adverse event report must contain at a minimum the following four elements
- One identifiable reporter
- One single identifiable patient
- One suspect medicinal product
- At least one suspected adverse event
The lack of any of these four elements means that the case is considered incomplete (non-valid) and does not qualify for regulatory reporting.
Any case report that does not meet these criteria must be further investigated and followed up to obtain the missing validity criteria.
Four minimum criteria are required to validate ICSRs.
-
Identifiable Reporter assessment criteria
The reporter should be identifiable and establish first-hand knowledge of the identifiable patient.
A reporter is considered identifiable if one or more of the following are provided
- Name/initials
- address (e.g., reporter’s organisation, department, street, city, state or province, postcode, country, phone number).
- Qualification (e.g., physician, pharmacist, other healthcare professional, lawyer, consumer or other non-healthcare professional)
- Email address
The reporter may be one of the following:
- Healthcare professional (HCP)
A healthcare professional is defined as a medically qualified person, such as a doctor, dentist, pharmacist, nurse, coroner or any other person designated by local regulations.
- Consumer (The consumer is defined as someone who is not a health professional such as a patient, lawyer, friend or relative, parent, child of a patient.
- Literature article Author
- Regulatory Authority
- Clinical study investigator/investigational site staff
For the reporter to be considered identifiable, contact details need to be available in order to confirm or follow-up the case, if necessary.
If a reporter does not wish to provide contact information, the ICSR must still be considered valid provided the organization who has been informed of the case was able to confirm it directly with the reporter.
Identifiable means that the notified organization has sufficient evidence of the existence of the person reporting the facts on the basis of the available information.
According to ICH E2B, an ICSR is not valid for the submission unless information about the qualification and country is available for at least one reporter.
-
Identifiable Patient assessment criteria
The following information qualifies as identifiable patient of which at least one condition is sufficient:
- Initials
- Patient identification number
- Medical record number (from general practitioner, specialist, hospital, or investigation)
- Age or age group for example, elderly or child
- gestation period
- Gender
- Date of birth (an incomplete date (year or month + year) should be considered as valid)
The information should be as complete as possible in accordance with local data protection laws
In accordance with the ICH-E2D standard, the term “identifiable” refers to the ability to verify the existence of a patient based on available information.
In case the patient is not identifiable (e.g., no patient demographics or multiple patients without any patient identifiers), but the reporter clearly mentioned a patient, then one case will be created (non-valid), even if the criteria of an identifiable patient are not fulfilled.
There is exception in Canada where case is valid.
If a report includes multiple patients experiencing an adverse event, but no specific patient number is provided (e.g., report states several patients) one non-valid case is created.
If the exact patient number is known, separate cases are created for each identifiable patient and considered as Valid case.
Reasonable attempts should therefore be made to obtain and submit the age or age group of the patient when a case is reported by a healthcare professional, or consumer in order to be able to identify potential safety signals specific to a particular population
Potential medication errors or near misses case:
Reports of medication errors that do not involve the patient’s exposure to the suspect product are not required to meet the patient identification criteria.
However, when a report of a medication error involves patient exposure to the suspect product, the patient must be identifiable.
-
Suspect medicinal product
The report must name a medicinal product, medical device, or blinded therapy
Product should be registered, in licensed, or co promoted by company
When the identifiable product is a biologic, biosimilar or vaccine, efforts are made to also include in the report information on trade name, batch/lot number and expiry date to enable characterization of the suspect product.
-
At least one suspected adverse event
The report should include at least one event such as signs or symptoms, a diagnosis or circumstances that increase the risk of an event.
A number of special scenarios are considered ‘nonreportable’ which are
- Lack of efficacy for an unapproved indication without an associated adverse event
- Medication error report without an associated adverse event
- Lack of efficacy reported with expired drug without an adverse event
- Drug exposure during pregnancy
- Lactation without an adverse event
A case will be created as ‘valid’ because these reports may not require or qualify for regulatory reporting but will be considered during signal detection activities.
If the primary source has made an explicit statement that a causal relationship between the medicinal product and the adverse event has been excluded and the recipient (competent authority or marketing authorisation holder) agrees with this, the report does not qualify as a valid ICSR since the minimum information is incomplete.
Additionally, the report does not qualify as a valid ICSR if it is reported that the patient experienced an adverse reaction but there is no information provided on the specific type of adverse reaction experienced.
Case reports only including unspecified event(s) will be entered on the safety database and considered as Non-valid
Non reportable events are If an adverse event is reported as surgery, procedure or hospitalization only with no other adverse events or diagnosis as this does not meet the criteria for a valid cas
Literature Reports (cases) Validity Criteria
Literature reports are considered valid only if they contain at least one adverse event identified by the publication’s author as having a reasonable possibility to be related to a company product.
For literature reports, the author is the identifiable reporter regardless of the direct knowledge of the identifiable patient.
Lay Media Reports (Cases) Validity Criteria
Information concerning adverse events from lay media and other sources, such as blog sites on the internet, do not meet criteria for valid reports, unless an identifiable reporter with firsthand knowledge of the patient is identified. The reporting source must identify the individual reporter.
When collecting reports of suspected adverse reactions via the internet or digital media, the term “identifiable” refers to the possibility of verification of the existence of a reporter and a patient (e.g., an email address under a valid format has been provided).
Invalid ICSR or non-valid or incomplete case
The lack of any of these four elements means that the case is considered incomplete (non-valid) and does not qualify for regulatory reporting
These cases should be considered as invalid.
Invalid cases should be created in Safety database if an event/special situation and the suspect drug were provided.
It is important that such cases are updated in Safety database in order to ensure ongoing safety evaluation activities.
Follow-up procedures should be performed to obtain missing information.
- If the patient is not identifiable but the reporter clearly mentioned a patient, then an invalid case should be created as the criterion of an identifiable patient has not been fulfilled.
- If only an outcome, a consequence or a special situation has been reported and no further information about the clinical circumstances has been provided, this is classified as non-AE. These reports should be flagged as invalid
- Medical judgment should always be used to decide whether the reported information is an adverse reaction or an event.
For example, a report of sudden death should usually need to be considered as a case of suspected adverse reaction and the valid ICSR should be submitted.
Non-valid cases do not require medical review.
Spontaneous and literature reports regarding a series of unidentifiable patients, which are entered as one case and suppressed until further information is available.
Cases where the only reported “event” is that the patient was hospitalised or had a surgery/procedure, with no further information provided.
Cases (mainly from ‘blogs’ or internet sites) that contain an e-mail address which does not include the subject’s name or institution as part of the email address
If a non-valid case becomes valid based on additional information received, then create a new version of the case in the database