ICSR Sources

ICSR Sources
Individual Case Safety Report (ICSR) refers to the format and content for the reporting of one or more adverse events in relation to a medicinal product that occur in a single patient at a specific point of time.
ICSRs may originate from various sources. Depending on the source and other factors, ICSRs can be categorized as
- Solicited sources – Received as a result of targeted data collection (i.e., establish a channel to collect the Adverse effects)
- Unsolicited sources – Received without request or Spontaneous in nature
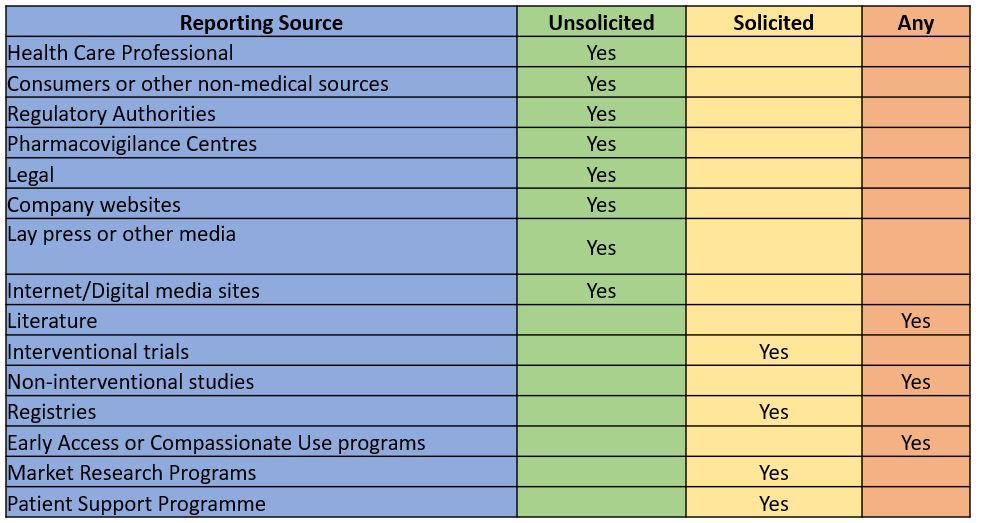
ICSR Sources: Solicited Reports
Solicited reports of suspected adverse reactions are those derived from organized data collection systems, which include
- Interventional trials
- Non-interventional studies
- Registries
- Early Access or Compassionate Use programs
- Market Research Programs
- Patient Support Programs
Interventional studies
Interventional trials include company-sponsored studies (Phase I – III) as well as studies sponsored by license partners for co-development programs
Non-interventional studies (NIS)
Non-interventional studies are a type of organized data collection
No study-related intervention is performed on the patient in NIS
NIS defines as a study where the medical product(s) is prescribed independent of the inclusion of the participant in the study and as part of a therapeutic strategy, including diagnostic and monitoring procedures, which is not decided in advance by a study protocol but is applied according to the current clinical practice.
As such, these studies seek to understand the use of a marketed product in real-world conditions, including risk/benefit, healthcare resource utilization, and patient/caregiver satisfaction, as examples.
Source: Article 2 of DIR 2001/20/EC
NIS may be Solicited or unsolicited
NIS – Solicited Reports
Where adverse events are actively collected from non-interventional studies, such ICSRs will be considered solicited.
Adverse events reported from non-interventional post-approval studies should be entered into the Company safety database as outlined in the protocol.
Non-serious adverse drug reactions should be entered when not excluded as per protocol.
NIS – Unsolicited Reports
For primary data collection studies for Adverse events (AEs) which per protocol are defined as not requiring active collection: HCPs and consumers must be informed in the protocol to report AEs according to local regulations, and to include the protocol number in the report.
Where such ICSRs are received by the company they will be treated as unsolicited.
Adverse events received spontaneously from participants enrolled in these studies, and which are not required as per protocol, should be captured as spontaneous reports.
Registry
An organized program that uses observational methods to collect uniform data on specified outcomes in a population defined by a particular disease, condition, or exposure.
Early Access or Compassionate Use programs
These programs are also known by various other names such as compassionate use, early access, special access, etc.
These programs along with clinical trials provide pre-launch access to the investigational medical product (drug, biologic, or medical device).
Making a medicinal product available for compassionate reasons to a group of patients with
- a chronically or seriously debilitating disease or
- whose disease is considered to be life-threatening, and
- who cannot be treated satisfactorily by an authorized medicinal product
Market Research
A market research program refers to the systematic collection, recording, and analysis by a marketing authorization holder or their subcontractor of data and findings of its medicinal products, relevant for marketing and business development.
For example, Surveys of patients or healthcare providers
Patient Support Programme
Patient Support Programme also called Patient assistance, and disease management programmes
The company sponsors Patient Support Programmes in the USA and other countries that provide approved products free of charge to indigent subjects who would otherwise be unable to benefit from the drug.
Once the patient has been approved for such a program, they are often contacted for re-enrolment into the program.
ICSR Sources: Unsolicited Reports (Spontaneous reports)
A spontaneous report is an unsolicited notification that describes one or more suspected adverse events in a patient who was given one or more medicinal products and does not derive from a study or any organized data collection systems where adverse event reporting is actively sought.
Spontaneous reports include all serious and non-serious Adverse events from
- Health Care Professional
- Consumers or other non-medical sources
- Regulatory Authorities
- Pharmacovigilance Centres
- Legal
- Company websites
- Internet/Digital media sites
- Literature
All ICSRs received from one of the above-mentioned reporting sources are considered suspected adverse reactions as the suspicion of a causal relationship between the drug and the event is implied.
Healthcare Professional/Consumer/ other non-medical sources
A Healthcare professional is defined as a medically-qualified person such as a physician, dentist, pharmacist, nurse, coroner, or as otherwise specified by local regulations.
The consumer is defined as a person who is not a healthcare professional such as a patient, lawyer, friend, or relative of a patient.
An unsolicited communication, including pre-publication manuscripts, by an HCP or consumer that describes one or more ADRs in a patient who was given one or more company products, and that does not derive from a study or any organised data collection scheme, is considered to be spontaneous.
Stimulated reporting that may occur following a Direct Healthcare Professional Communication, publication in the press, questioning of an HCP by a company representative, or class action lawsuits must be considered spontaneous reports for the purposes of case handling.
Non-medical sources, e.g., the lay press or other media, it should be handled as a spontaneous report
Regulatory Authority Sources/ Pharmacovigilance Centres
In some countries reports of ADRs are sent directly by HCPs and consumers to the Regulatory Authorities or to Pharmacovigilance (PV) Centres.
Where reports are received from authorities or PV Centres, the company must make reasonable efforts to identify any duplicates, for example, ICSRs that may previously have been reported by an HCP directly to the company or to the local Regulatory Authority.
Legal cases
Legal sources, such as a class lawsuit, have the potential to include ADRs involving company products.
Any ADRs from this source is considered to be unsolicited and so are treated as spontaneous reports.
Company websites
Companies should consider using their websites to facilitate the collection of Adverse drug reaction (ADR) data, such as providing ADR forms for reporting or providing appropriate contact information for direct communication.
Internet/Digital media sites
Companies should regularly review websites under their direction or responsibility for possible ADR case reports.
It is not expected that Companies will review external websites for ADR information.
However, if a Company becomes aware of an adverse reaction on a website that the company does not manage, the company must review the case and determine if it needs to be reported.
Literature
As per ICH, Reports of suspected adverse reactions and reports of special situations from scientific and medical literature, including relevant published abstracts from meetings and draft manuscripts, should be reviewed and assessed by marketing authorization holders to identify and record ICSRs originating from spontaneous reports or non-interventional post-authorization studies.
Literature Solicited Reports
Literature searches include publications involving company products used in interventional/non-interventional studies.
ADRs identified from articles that specifically describe the use of the company product in a study are considered to be solicited.
Literature Unsolicited Reports
All other ICSRs (not referencing a study source) identified from publications are considered to be unsolicited.
Other articles related to ICSR
2 thoughts on “ICSR Sources”