Adverse reaction reporting in New Zealand (2020)

Adverse reaction reporting in New Zealand (2020)
Adverse reaction reporting in New Zealand (2020)
On 22 April 2021, Med Safe published 2020-year Adverse reaction reporting statistics in New Zealand.
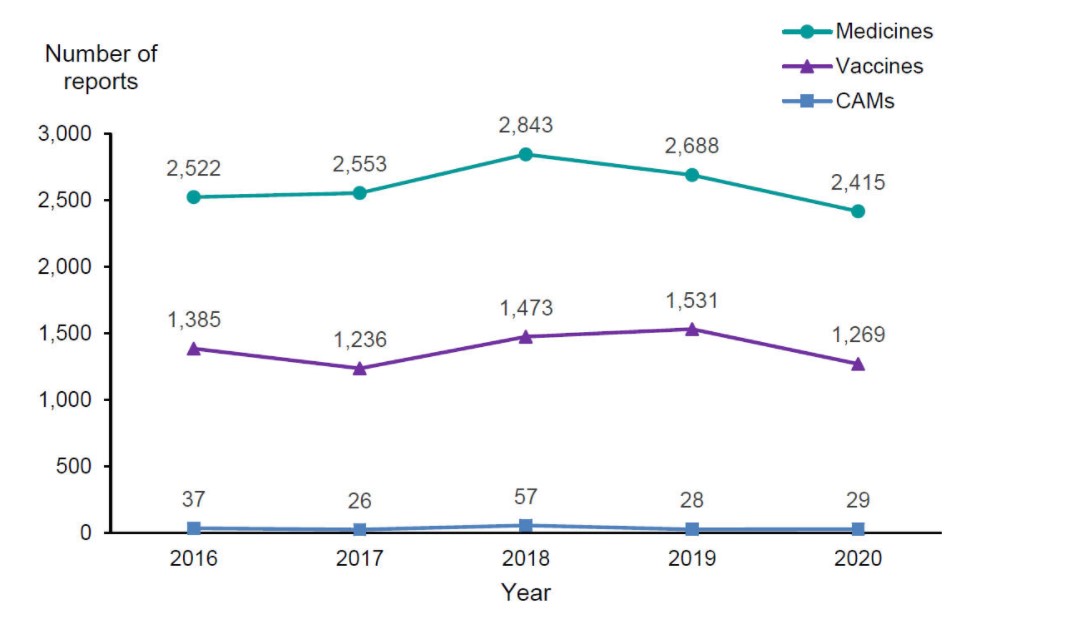
In 2020, the Centre for Adverse Reactions Monitoring (CARM) received a total of 3,713 reports of suspected adverse reactions. These included 2,415 reports associated with medicines, 1,269 reports associated with vaccines, and 29 reports associated with complementary or alternative medicines.
Of all reports received in 2020, 20.7 percent were considered serious. Serious reports accounted for 29.6 percent of medicine reports, 3.6 percent of vaccine reports and 34.5 percent of CAM reports. A serious adverse reaction is defined as any reaction that results in death or is life-threatening, causes or prolongs hospitalisation, results in persistent or significant disability/incapacity, is a congenital abnormality or is a medically important event.
You can find more information about suspected adverse reactions reported in New Zealand on the Medsafe website, using the Suspected Medicines Adverse Reaction Search (SMARS)
Adverse reaction reporting in New Zealand (2020)
For More information on Adverse reaction reporting in New Zealand (2020) @ Click Here
Top Related Articles:
Pfizer-biontech-covid-19-vaccine-safety-data
FDA-briefed-moderna-covid-19-vaccine-safety-data
Oxford-University-Astrazeneca-covid-19-vaccine-safety-data
Safety concerns-of Covid-19 vaccines
FDA alerts potential risks associated with the compounding of Remdesivir drug products
Risk of thrombocytopenia and coagulation disorders with the use of COVID-19 Vaccine AstraZeneca
Safety signal (risk of thrombosis in combination with thrombocytopenia) VAXZEVRIA/COVID-19 Vaccine
Increased risk of angioedema with vildagliptin and ACE inhibitors
Great content, thank you for your information… i shared a link here, its related to this topic. i hope it will also helpful to us: https://www.vistaar.ai/blogs/adverse-reaction-reporting-guide-for-new-zealand