CIOMS role in Pharmacovigilance

CIOMS role in Pharmacovigilance
CIOMS full form is Council for International Organizations of Medical Sciences
CIOMS is an international, non-governmental, non-profit organization
CIOMS plays a vital role in Pharmacovigilance Process.
In 1949, CIOMS established jointly by WHO and UNESCO
The CIOMS is based in Geneva, Switzerland, and operates under the WHO umbrella.
It has served as a forum for discussions between regulators and industry on a variety of pharmacovigilance topics since the late 1980s.
Over the years, reports from CIOMS working groups have been highly influential in shaping legislation and guidelines around the world.
CIOMS Working Groups usually take two to four years to finalize their guidance and recommendations.
These groups have published many Guidelines for Pharmacovigilance practice
Here we are summarizing formal CIOMS working group topics and the broad vision of each group
Table of Contents
CIOMS role in Pharmacovigilance: CIOMS Working Groups
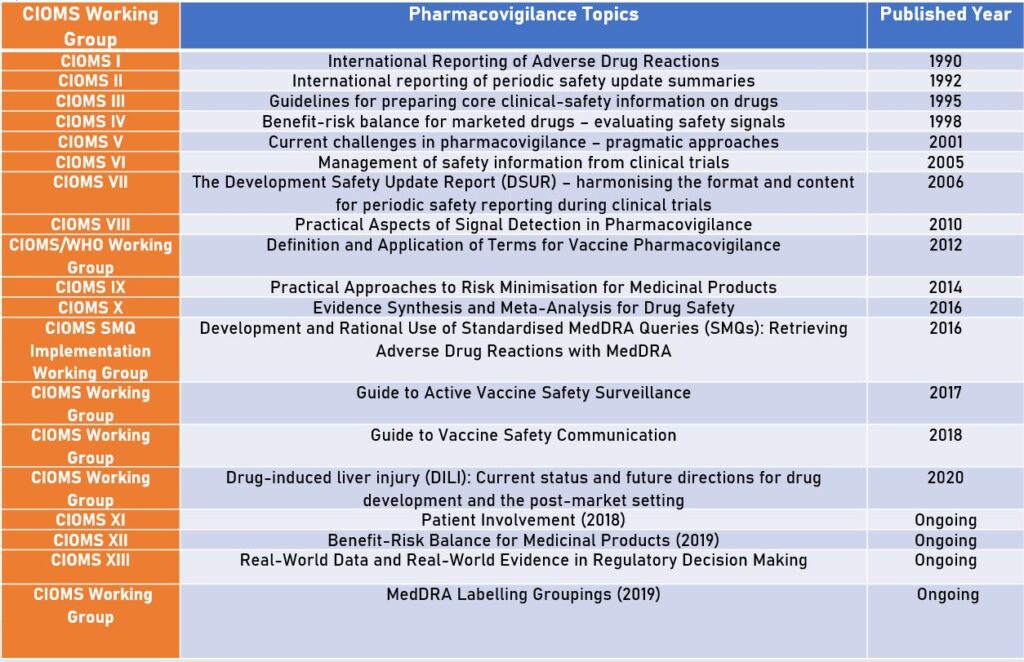
CIOMS I: International reporting of Adverse Drug Reactions (1990)
- The CIOMS has established a first working group on pharmacovigilance in 1986
- Prior to the mid-1980s, reporting requirements to regulatory authorities related almost entirely to the reporting of national Adverse drug reactions (ADRs), i.e., those that occurred within the country.
- Some countries then started to introduce requirements for submission of ‘foreign’ reports.
- This working group was called to discuss the principles of what should be reported and how it should be reported.
- The key output was that such reports should be of suspected reactions that were both serious and unexpected (i.e., unlabelled).
- The group also proposed that 15 days was the appropriate maximum time frame for submission and developing a reporting form – the ‘CIOMS form’ which became the international standard.
- In 1995, the working group reconvened (known as CIOMS Ia) and made proposals for the data elements to be included in the electronic transmission of reports.
CIOMS II: International reporting of periodic safety update summaries (1992)
- This report made the original proposals for the format and content of PSURs.
- Since 1992, requirements for PSURs have been widely implemented as a regulatory requirement and a harmonised guideline has been adopted through the International Conference on Harmonisation.
CIOMS III: Guidelines for preparing core clinical-safety information on drugs (1995)
- This report addressed the problem of variations in safety labeling around the world by proposing that manufacturers should develop a ‘core data sheet’
- The core data sheet contains all the relevant safety information which needs to be included in all countries where the drug is marketed.
- The report made specific recommendations as to what should be included in core safety information, and when and how it should be included.
CIOMS IV: Benefit-risk balance for marketed drugs – evaluating safety signals (1998)
This report proposes a standard format and content for a benefit-risk evaluation report and also lays down the principles for good decision-making practices.
The proposed structure of the report is as follows:
- Introduction
- Benefit evaluation
- Risk evaluation
- Benefit-risk evaluation
- Options analysis
CIOMS V: Current challenges in pharmacovigilance – pragmatic approaches (2001)
This report is about good case management and reporting practices, both in terms of individual cases and summaries.
It includes almost 20 pages of detailed and specific recommendations.
The general aim of the report is that, despite much progress on harmonization, much remains ineffective and that the ultimate goal should be a single, global shared data set.
CIOMS VI: Management of safety information from clinical trials (2005)
The aim of this report is to enhance awareness of the ethical and technical issues associated with safety in clinical trials.
It proposed a systematic approach to managing safety during clinical development and is
- wide-ranging,
- covering, e.g., ethical issues, statistical approaches to identifying risks and
- communication of safety information from clinical trials.
CIOMS VII: The Development Safety Update Report (DSUR) – harmonising the format and content for periodic safety reporting during clinical trials (2006)
This report proposes the content and format for a DSUR – a means of regular and timely review, appraisal and communication of safety information during the clinical development of drugs.
The working group anticipates that in the future the DSUR and PSUR could be incorporated into a single harmonized safety report that would cover a product throughout its lifecycle.
CIOMS VIII: Practical Aspects of Signal Detection in Pharmacovigilance (2010)
This working group is focused on signal detection and management activities
The working document contains
- Key definitions of pharmacovigilance
- Taxonomy of drug safety signals
- Description of approaches to signal detection including both traditional and statistical data mining methods and interpretation of results
- Data elements, mechanisms, and various data systems reported spontaneously, including discussions on limitations and challenges of spontaneous data, are presented
- Signal detection strategies and program, stakeholder perspectives and
- Signal management process
- Elaborated on future directions in signal detection, evaluation, and communication
CIOMS role in Pharmacovigilance: Definitions and Applications of Terms for Vaccine Pharmacovigilance (2012)
The Working Group established in November 2005 at the request of WHO to:
Develop general definitions strictly focused on Vaccine Pharmacovigilance
Contribute to the assessment of the development review and approval of the definitions of adverse events following immunization as developed by the process Brighton Collaboration (BC) and their release:
- In endorsing already existing definitions
- In participating in the review of definitions under development
- In proposing priorities for the development of new definitions
- Facilitating the translation and dissemination of the definitions
CIOMS IX: Practical Approaches to Risk Minimisation for Medicinal Products (2014)
This group created in 2010 based on suggestions from drug regulatory authorities and pharmaceutical companies.
CIOMS I – VIII working groups have provided guidance on certain essential risk management components, such as
- terminology and adverse drug reaction reporting,
- managing information on the safety of clinical trials and
- the detection of safety signals
Published CIOMS WG IX report in August 2014.
This group addressed the aspect of minimizing the risks of medicines.
It covers the prevention and mitigation of risk
CIOMS X: Evidence Synthesis and Meta-Analysis for Drug Safety (2016)
Meta-analyses are increasingly used in the scientific evaluation of effectiveness and safety in benefit-risk assessments
This group published a report on Considerations for applying good meta-analysis practices to clinical safety data within the biopharmaceutical regulatory process
Development and Rational Use of Standardised MedDRA Queries (SMQs): Retrieving Adverse Drug Reactions with MedDRA (2016)
This group started in 2002 in response to indications received from some drug regulatory authorities and pharmaceutical companies that they had concerns about the parallel development of special drug safety search programs based on the Medical Dictionary for Regulatory Activities (MedDRA).
The pharmaceutical companies and regulatory authorities identified a need to harmonize and standardize their adverse drug reaction database search queries based on MedDRA
In 2003, a Memorandum of Understanding between CIOMS and the International Federation of Pharmaceutical Manufacturers Associations (IFPMA) was drafted regarding the SMQ project.
Since 2002 the CIOMS Working Group on Standardised MedDRA Queries (SMQs) has developed search queries for over 100 selected adverse drug reactions.
In 2016, the Implementation Working Group completed its second edition of Development and Rational Use of Standardised MedDRA Queries (SMQs): Retrieving Adverse Drug Reactions with MedDRA
Active Vaccine Safety Surveillance (2017)
This group established in 2013
The main objectives of this group were:
- Promote more effective and rapid information gathering and exchange between national regulatory agencies, multilateral agencies, and vaccine manufacturers
- Develop guidance documents on the use of harmonized tools and methods for vaccine safety oversight activities among national regulators, multilateral agencies, and vaccine manufacturers
- Propose vaccine safety oversight mechanisms in challenging contexts, i.e., those with minimal infrastructure
CIOMS Guide to Vaccine Safety Communication (2018)
This guide provides an overview of the strategic communication challenges facing drug regulators, vaccine policy and program officials, and other stakeholders when dealing with:
- the entry into the market of newly developed vaccines for the first time
- the introduction of existing or under-utilized vaccines in new countries, regions or populations
- the management of any new safety issues that occur over the life cycle of a vaccine
Click on for template for creating a vaccine communications plan.
Drug-induced liver injury (DILI): Current status and future directions for drug development and the post-market setting (2020)
First meeting held in April 2017
The main objective of this group is to address the present knowledge and practice gaps related to drug-induced liver injury
This working group provided guidance on
- General Classification of DILI
- DILI Phenotypes
- DILI in Patients with Pre-existing Liver Disease
- DILI Detection
- Risk Assessment in Drug Development
- Summary
CIOMS XI: Patient Involvement
This group has created in 2018
This group is working on patient involvement in the development and safe use of medicines
CIOMS XII: Benefit-Risk Balance for Medicinal Products
This group is created in 2019
The main objective of this group is
To build on previous considerations established by CIOMS WG IV, incorporating the latest thinking in quantitative and qualitative approaches to the evaluation of benefit-risk
As well as assimilating visual presentations of benefits and risks to improve transparency and understanding amongst key stakeholders, including patients
CIOMS XIII – Real-World Data and Real-World Evidence in Regulatory Decision Making
There is growing interest in using Real-World (RWD) data to support regulatory decision-making throughout the product’s lifecycle.
RWD sources are electronic health records, claims data, prescription data, and patient registries.
Real-World Evidence (RWE) is the clinical evidence of the use and potential benefits or risks of a medical product arising from an RWD analysis.
CIOMS Working Group XIII is currently working on this report
The main objective of this Working group is
CIOMS guidelines would create a global reference for researchers and regulatory bodies involved in RWD/RWE studies, and for pharmaceutical and biotechnology firms involved in the development and marketing of products.
The task force would also be expected to evaluate the increasingly innovative methodologies used to design studies, collect and analyze data and use the output in the regulatory process to assess how RWE can contribute to effectiveness decisions.
MedDRA Labelling Groupings
First meeting held in April 2019
This working group is proposed to develop principles and pragmatic recommendations to promote consistency and understandability of medical concepts, e.g. suspected adverse reactions in biopharmaceutical product safety labeling
Source from CIOMS website. For More Info Click Here
One thought on “CIOMS role in Pharmacovigilance”