Update on Periodic Benefit-Risk Assessment Reports ANVISA_Brazil_Updated on 24 August 2021 Background: In 2009, the first pharmacovigilance legislation at the national level […]
Blog Archive
Designated Medical Event (DME)
Designated Medical Event (DME) European Medicines Agency has developed the Designated Medical Event (DME) list to help to prioritize the review of […]
Analysis of similar events (AOSE)
Analysis of similar events (AOSE) The sponsor must identify in each IND safety report, all IND safety reports previously submitted to Food […]
Pharmacovigilance interview questions for freshers 2021
Pharmacovigilance interview questions for freshers 2021 What is Pharmacovigilance? Pharmacovigilance is the science of collecting, monitoring, researching, assessing and evaluating information from […]
Adverse drug reactions
Definition of Adverse drug reaction Adverse drug reactions (ADR’s) are an important clinical problem. According to a World Health Organization (WHO), an […]
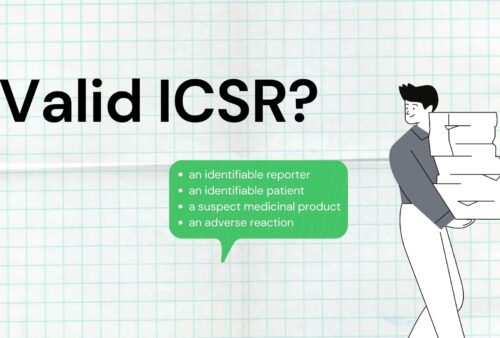
Valid ICSR?
Valid ICSR (Case) is called Potential ICSR (Individual case safety report). Only valid cases qualify for regulatory submission. Once receive a case […]
ICSR Sources
Individual Case Safety Report (ICSR) refers to the format and content for the reporting of one or more adverse events in relation […]
ICSR Process
ICSR i.e. Individual Case Safety Report ICSR Process is a part of the Pharmacovigilance Process. Individual Case Safety Report (ICSR) refers to […]
What is the Pharmacovigilance process?
What is the Pharmacovigilance process? Simply it is a drug safety monitoring process. Pharmacovigilance is one of the most important departments in […]