FDA Approved Novel Drugs for 2020 What is Novel Drug? A Novel Drug or a New Molecular Entity (NME) is an active, […]
Latest Articles
Read the latest release articles and learn without limits.
Oxford University/AstraZeneca COVID-19 vaccine Safety data
Oxford University/AstraZeneca COVID-19 vaccine Safety data: On 30 December 2020, the Medicines and Healthcare products Regulatory Agency (MHRA) approved the COVID-19 vaccine […]
ANVISA PBRER list 2021 (Brazil)
ANVISA PBRER list 2021 (Brazil) Background In 2009, the first pharmacovigilance legislation at the national level was published in Brazil. On 22 […]
Drug Safety Alerts UK MHRA (November 2020)
Drug Safety Alerts UK MHRA (November 2020): UK Medicines & Healthcare products Regulatory Agency (MHRA) has provided 8 safety letters and 06 […]
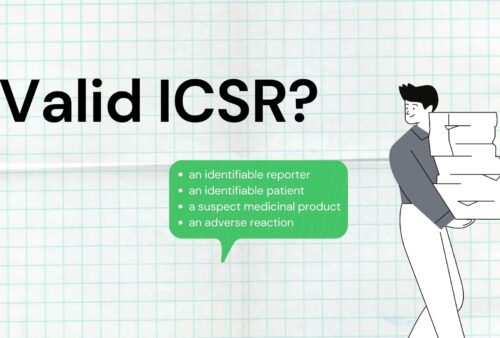
Valid ICSR?
Valid ICSR (Case) is called Potential ICSR (Individual case safety report). Only valid cases qualify for regulatory submission. Once receive a case […]
FDA briefed Moderna COVID-19 Vaccine Safety data
FDA briefed Moderna COVID-19 Vaccine Safety data FDA Issued Emergency Use Authorization (EUA) for emergency use of Moderna COVID-19 Vaccine for the […]
ICSR Sources
Individual Case Safety Report (ICSR) refers to the format and content for the reporting of one or more adverse events in relation […]
ICSR Process
ICSR i.e. Individual Case Safety Report ICSR Process is a part of the Pharmacovigilance Process. Individual Case Safety Report (ICSR) refers to […]
CIOMS role in Pharmacovigilance
CIOMS full form is Council for International Organizations of Medical Sciences CIOMS is an international, non-governmental, non-profit organization CIOMS plays a vital […]
What is the Pharmacovigilance process?
What is the Pharmacovigilance process? Simply it is a drug safety monitoring process. Pharmacovigilance is one of the most important departments in […]